Process for desulphurisation of liquid hydrocarbon fuels
a technology of liquid hydrocarbon fuels and desulphurization, which is applied in the petroleum industry, petroleum industry, and treatment with plural serial refining stages, etc., can solve the problems of difficult removal, fuels are receiving intense scrutiny, and high consumption inevitably has a major impact on the global environmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0080] A series of experiments were carried out to optimize sulphur to oxidant mole ratio, carboxylic acid to hydrocarbon ratio and reaction time. All these experiments were carried out in a jacketed mixer settler equipped with mechanical stirrer, thermowell, neck for addition of reactant and drain valve. The temperature of reactants was maintained at desired level by circulating hot fluid in the jacket of the mixer settler. In the general experimental procedure, HDS diesel feedstock (I) (100 ml, 83.34 g) was added to the mixer settler and stirrer, hot fluid were started to keep the temperature of the reactant at 50° C. Desired amount of formic Acid (99-100%) was then added to the diesel followed by addition of fixed amount of 30% aqueous Hydrogen peroxide. The mixture was then stirred vigorously at 50° C. for a certain period and then taken out in the separating funnel through drain valves. The two layers namely hydrocarbon layer and formic acid layer were then separated. The diese...
example 2
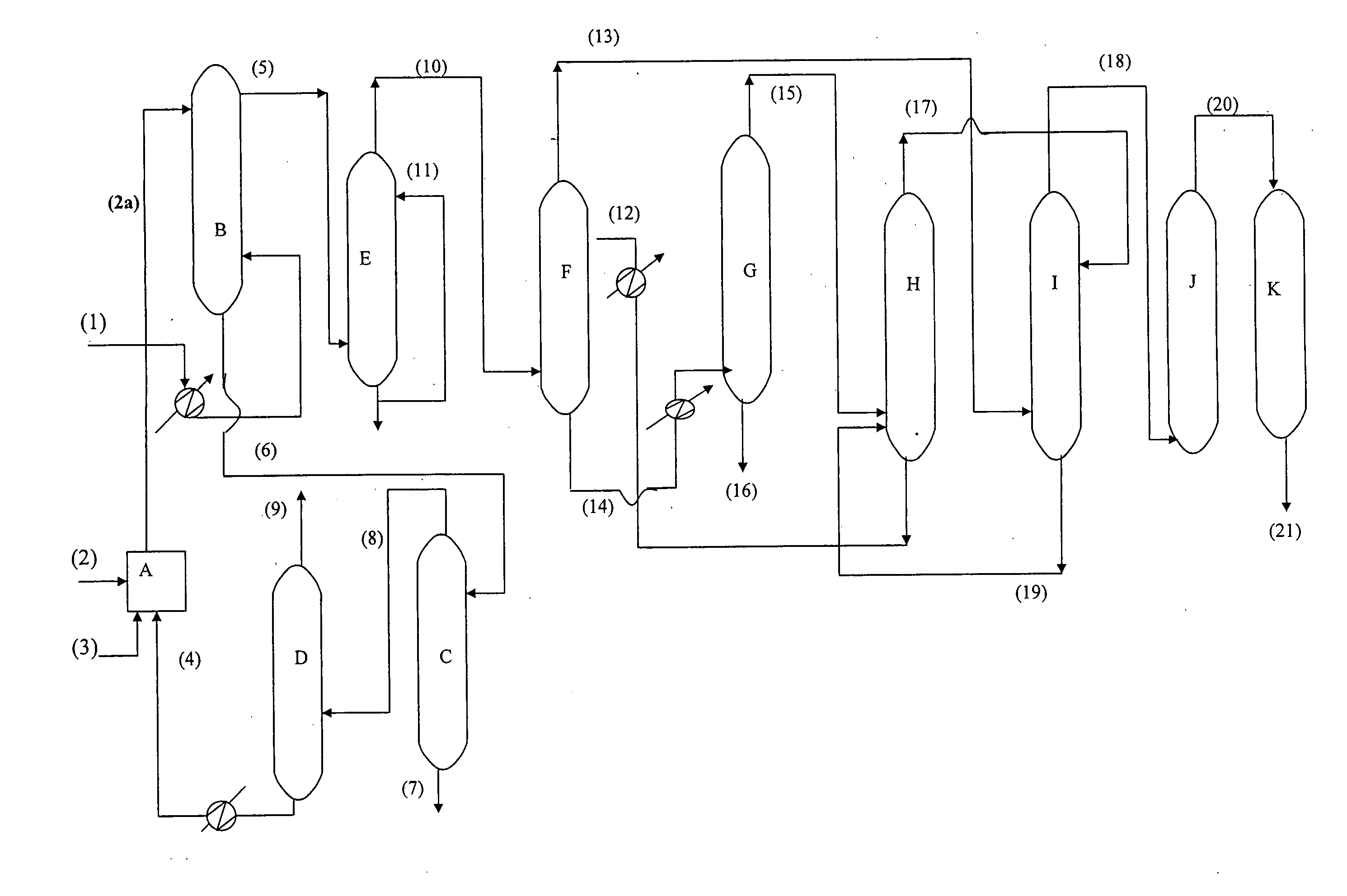
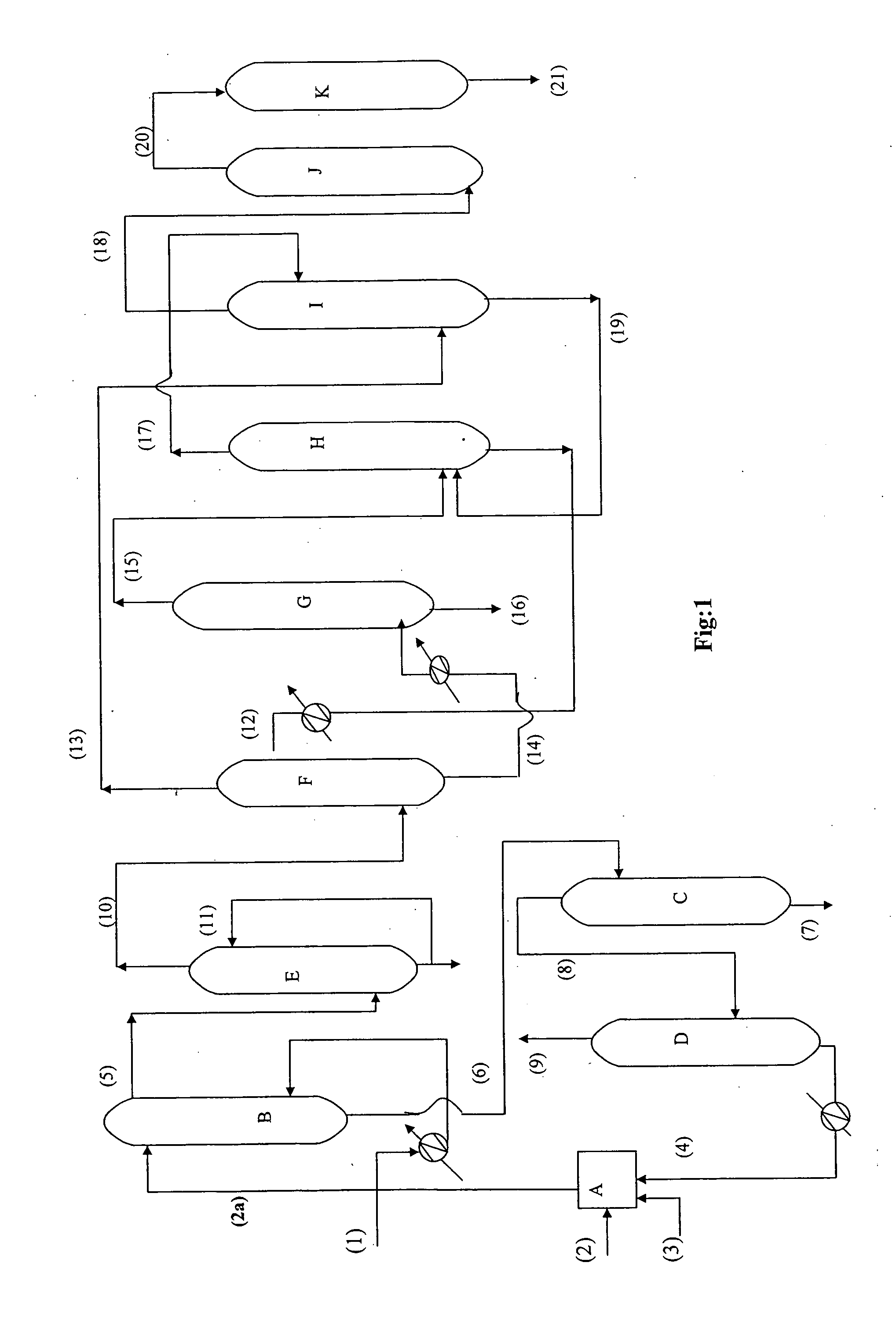
[0082] After establishing the reaction parameters in mixer settler, experiments were carried out on oxidative desulphurization using HDS diesel feedstock (I) as feed as per the scheme shown in FIG. 1 in a blockout mode. The general discussion regarding FIG. 1 is given in Detailed description of the process. The oxidation of HDS diesel feedstock (I) was carried out in continuous counter current oxidation column B by using diesel as continuous phase at a flow rate 0.59 kg / hr and formic acid, 30% aqueous hydrogen peroxide mixture at a flow rate 0.29 kg / hr as disperse phase. The temperature of the continuous counter current oxidation column was maintained at 70° C. by passing hot water in the jacket of the column. The characteristics of the oxidized diesel obtained after acid neutralization column are given in Table 3. As seen from the Table 3 while there was no change in the cetane index of the HDS diesel after oxidation, the total sulphur present got reduced from 437 ppm to 365 ppm du...
example 3
[0087] Oxidative desulfurization of HDS diesel from another refinery (Feedstock II) was carried out in similar manner as described in Example 2 in a blockout mode. The oxidation of HDS diesel feedstock (II) was carried out in a continuous counter current oxidation column B by passing diesel as continuous phase at flow rate 0.59 kg / hr. and formic acid, 30% aqueous hydrogen peroxide mixture at a flow rate 0.29 kg / hr as discrete phase at 50° C. Characteristics of oxidised diesel obtained after and neutralization step is given in Table-5
TABLE 5CHARACTERISTICS OF OXIDIZED DIESEL FEEDSTOCK(II)Oxidized DieselCharacteristicsFeedstock (IT)R.I at 20° C.1.4678Density at 20° C., g / cc0.8446Aromatics wt % (ASTM 2549)26.2Total sulphur452 ppmCetane index53.7ASTM D 86IBP166 5% Recovered, ° C.211.810% Recovered, ° C.240.650% Recovered, ° C.293.990% Recovered, ° C.351.0FBP375.0Class type analysis wt %Total saturates72.6Mono AromaticsNDPoly AromaticsNDTotal Aromatics27.4
ND = not determined
[0088] Agai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com