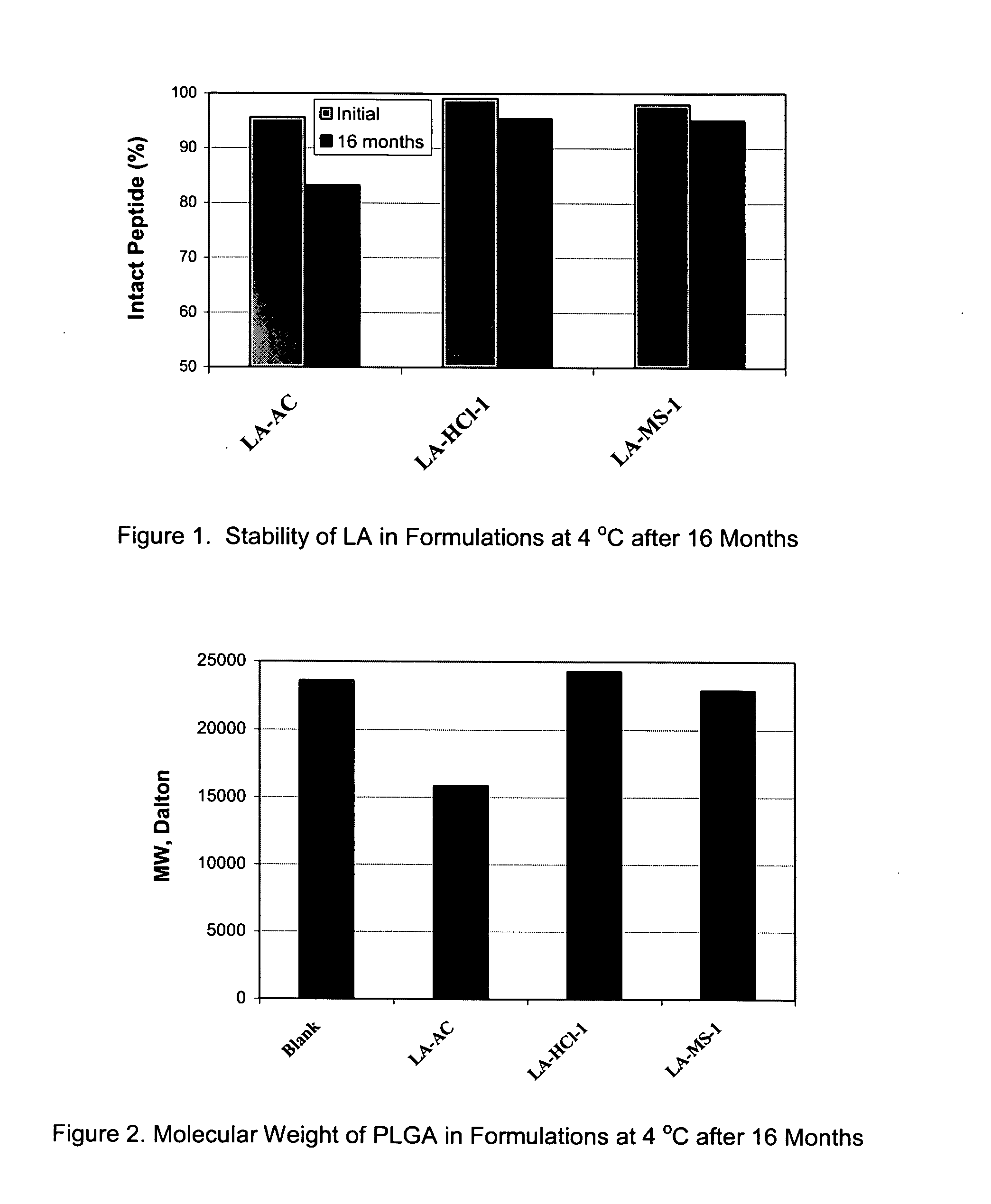
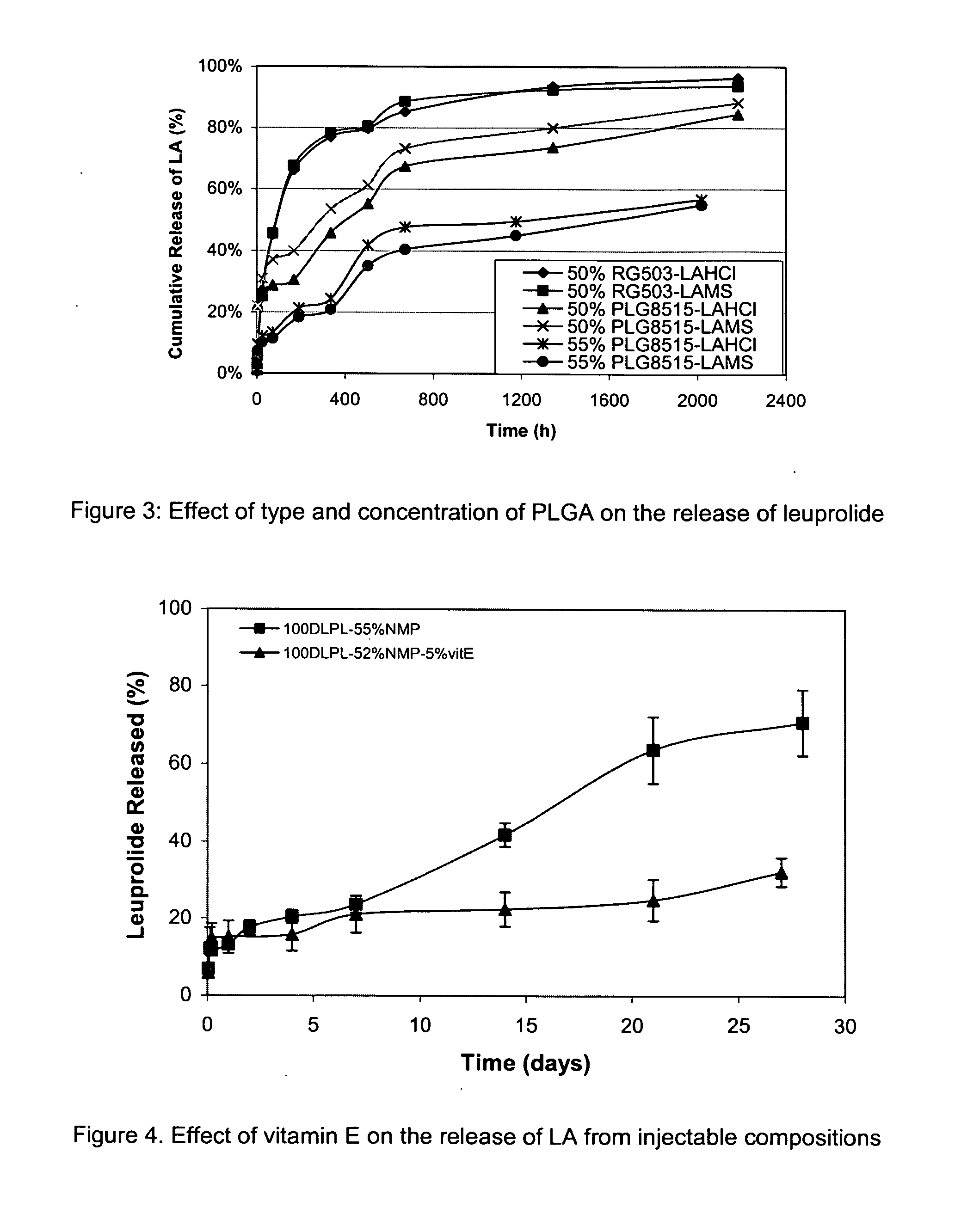
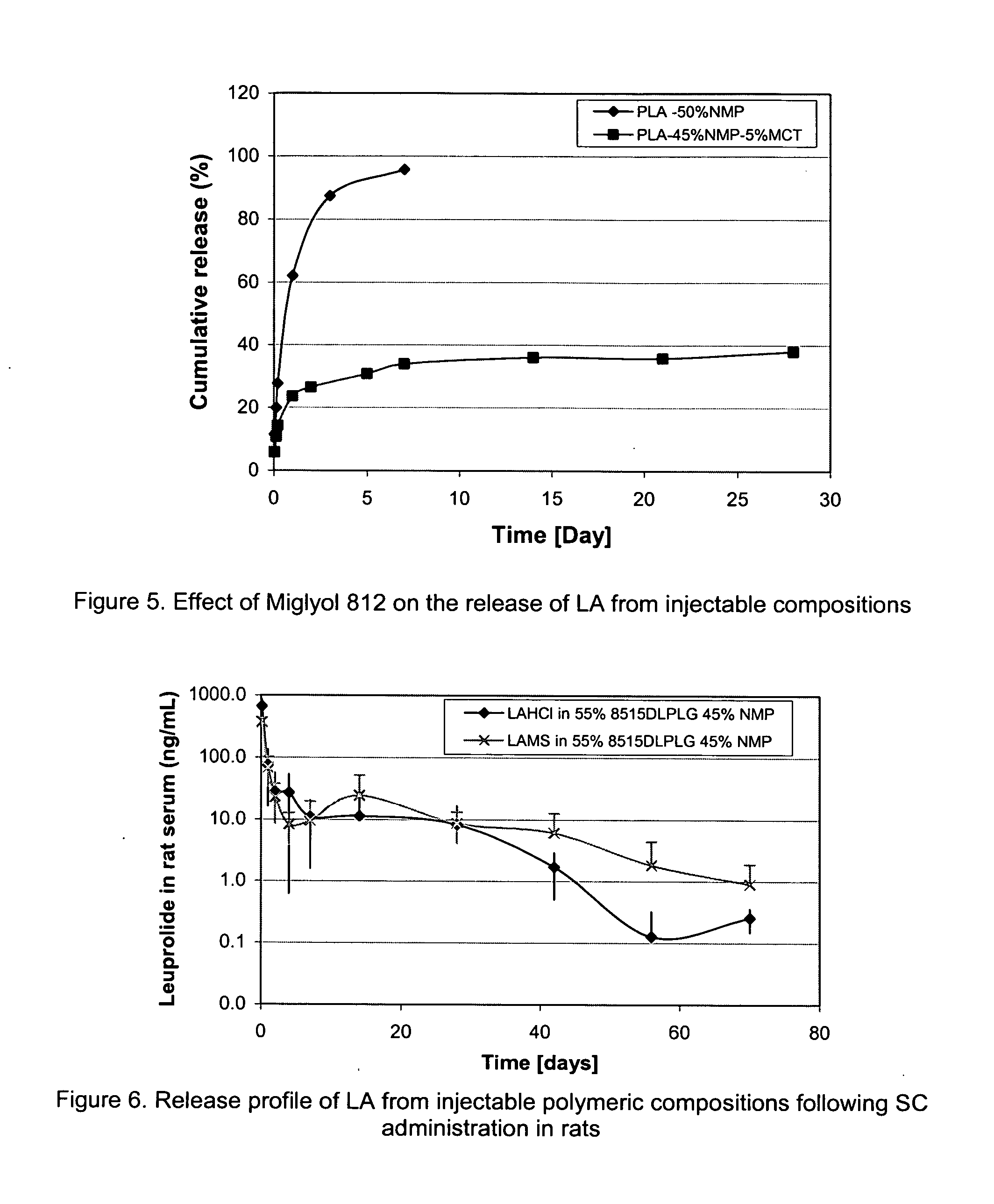
Pharmaceutical compositions with enhanced stability
a technology of compositions and stability, applied in the direction of peptide/protein ingredients, powder delivery, aerosol delivery, etc., can solve the problems of inability to meet the needs of use, inconvenient storage, and long half-life of peptide agents, so as to achieve satisfactory storage stability, minimize the interaction/reaction between peptide agents and polymers, and improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Beneficial Salts of Peptide Agents and Peptide Derivatives Formed with Strong Acids
[0095] Peptide agent or peptide derivative containing at least one basic functional group is dissolved in water. Stoichiometric amounts of a strong acid are added to the aqueous solution of the peptide agent, resulting in neutralization of the basic groups in the peptide agent. The salt is obtained by precipitation, filtration and / or lyophilization.
example 2
Preparation of Leuprolide Hydrochloride
[0096] Leuprolide is a luteinizing hormone releasing hormone (LHRH) agonist containing 9 amino acid residues and two basic functionalities (a histidine and an arginine group). Its N-terminal amine was blocked in the form of pyroglutamic acid. It has been used in the treatment of prostate cancer and endometriosis. Leuprolide acetate (LA-Ac) was obtained from Polypeptides Laboratories, Inc. (PPL Lot#PPL-LEUP0401A). Leuprolide Hydrochloride (LA-HCl) was prepared by replacing acetic acid with HCl through an ion-exchange and lyophilization process. Typically, 1000 mg of leuprolide acetate was dissolved in 30 mL water. 3.19 mL of 0.5 N HCl (HCl:LA˜2.2:1) was added and mixed well. The solution was freeze-dried for 72 h to remove acetic acid. The dried powder was re-dissolved in water and freeze-dried again.
example 3
Preparation of Leuprolide Mesylate
[0097] 343.5 mg of leuprolide acetate (PPL Lot#PPL-LEUP0401A) was dissolved in 20 mL water. 32 μL of methanesulfonic acid was added and mixed well (molar ratio of leuprolide acetate to methanesulfonic acid ˜1:2). The solution was freeze-dried for 72 h to remove acetic acid. The dried powder was re-dissolved in water and freeze-dried again.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight average molecular weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com