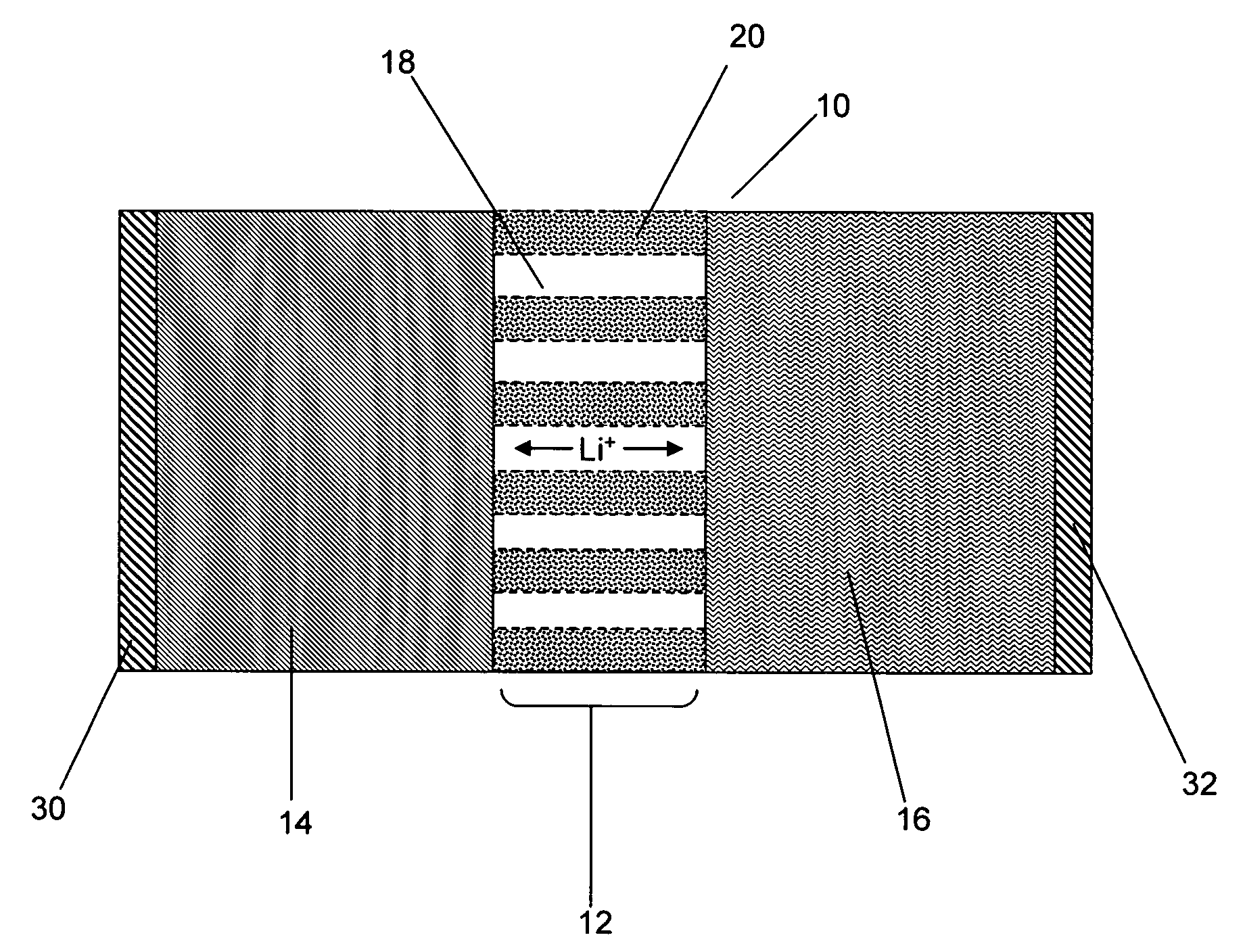
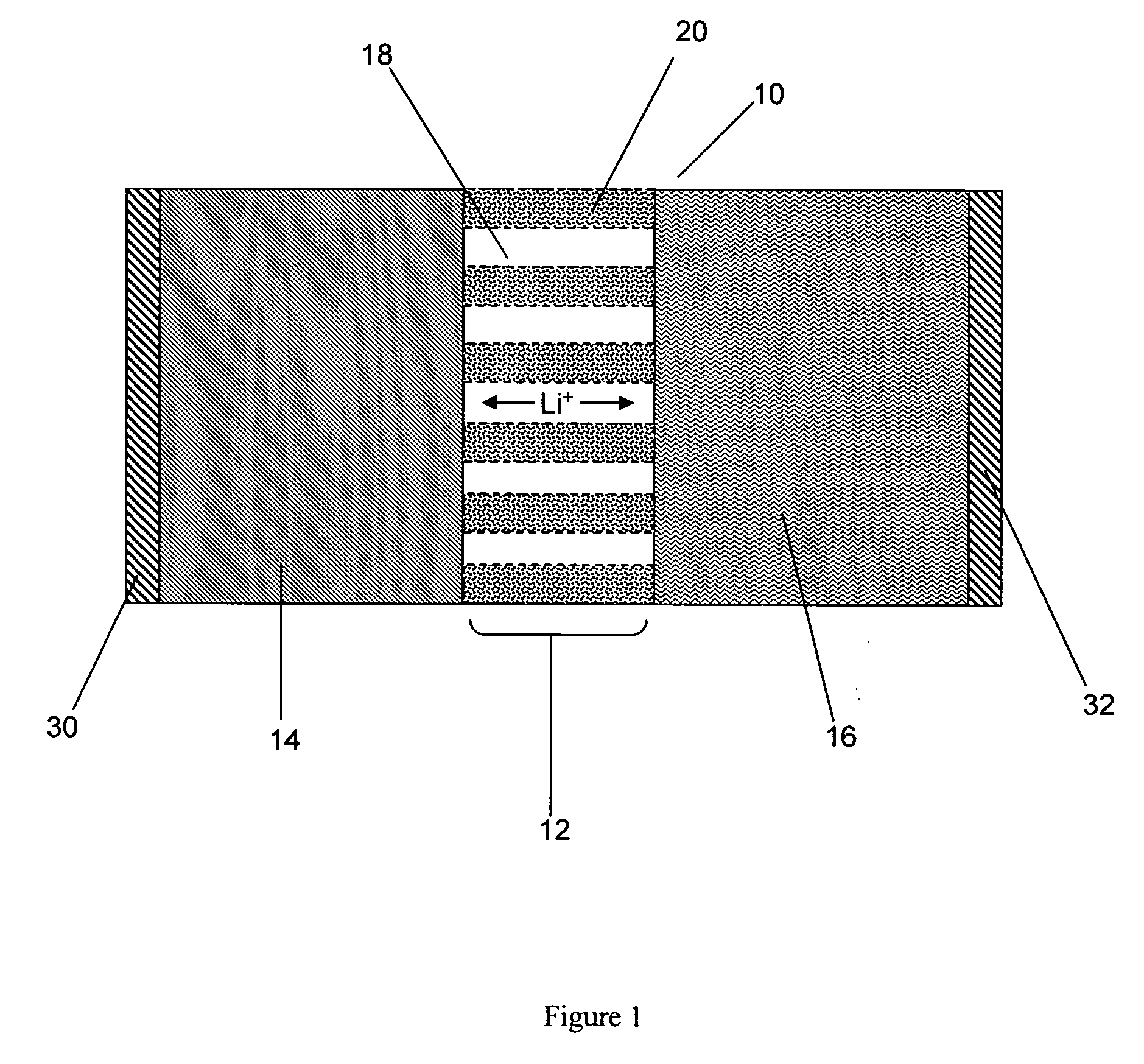
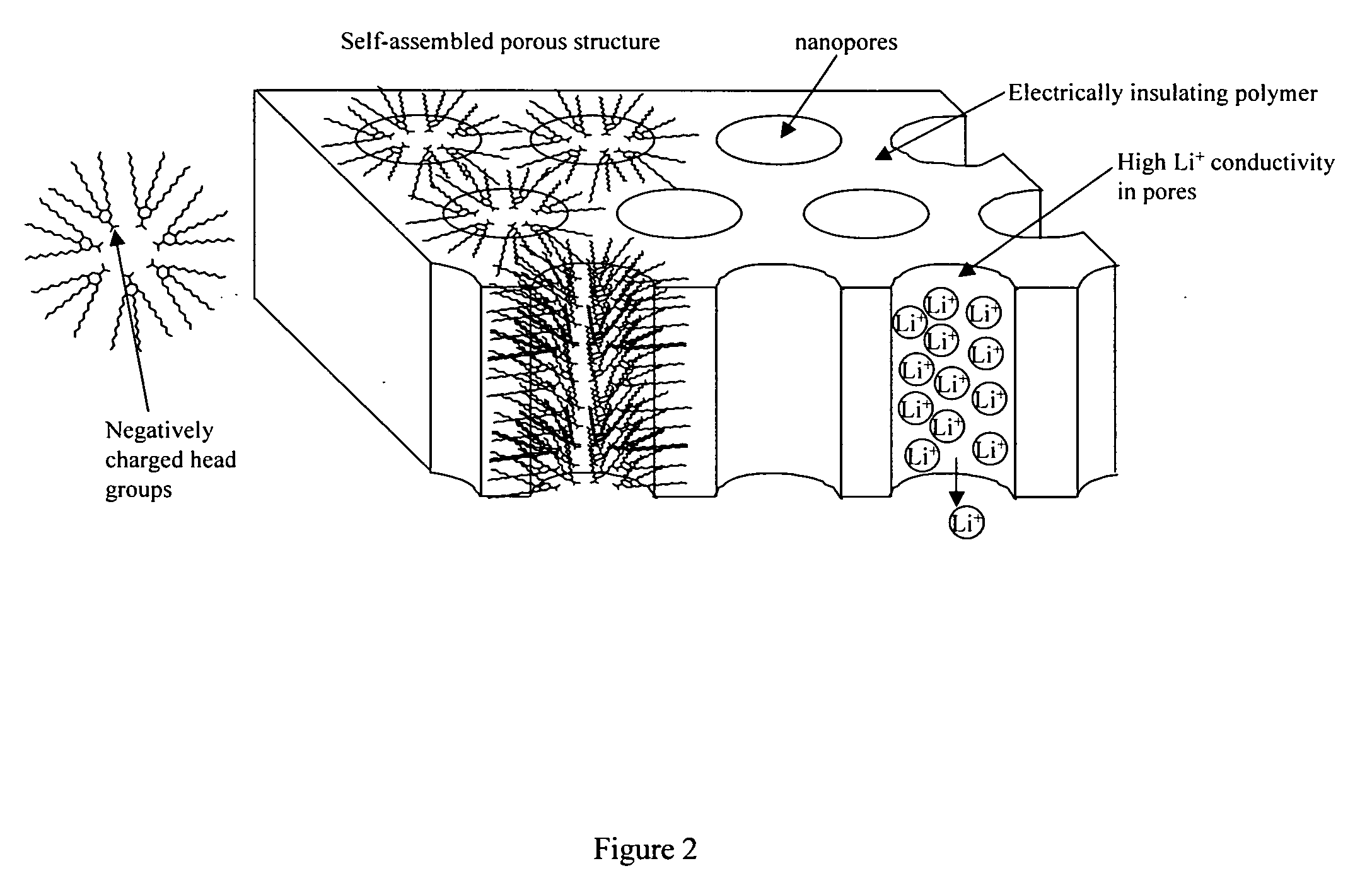
Nanoporous polymer electrolyte
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Lithium Sulfanilate Salt Monomer
[0081] This example details the preparation of a polymerizable surfactant containing a lithium salt headgroup, which may be used to make polymer lithium electrolytes.
Preparation of Acid Chloride Precursor
[0082] First, an acid chloride is prepared as depicted in step 1 of FIG. 1. All glassware was heated in an oven at 110° C. for 2 hours, and all anhydrous solvents were further dried with molecular sieves and purged with argon for 10 min, prior to the synthesis. First, 3,4,5-tris(11′-acryloyloxyundecyloxy)benzoic acid (1.09 g, 1.3 mmol) and a trace amount of 2,6-di-tert-butyl-4-methylphenol stabilizer were dissolved in anhydrous CH2Cl2 (25 mL) in a 50 mL Schlenk flask under an argon atmosphere. Thionyl chloride (0.57 mL, 7.8 mmol) was then injected by syringe directly into the solution with constant stirring. The flask was covered with aluminum foil, and the mixture was continually stirred for an overnight.
[0083] The solvent and the ...
example 2
Preparation of a Sodium Sulfanilate Headgroup Polymerizable Surfactant
[0087] This sodium sulfanilate surfactant is a variation of the lithium polymerizable surfactant in Example 1. The XRD spectrum of this sulfanilic acid polymerizable surfactant was used to help identify the phase structure for the material in Example 1.
[0088] The acid chloride precursor was identical to the compound described in step 1 of Example 1. To prepare the sodium sulfanilate salt monomer, the prepared acid chloride oil was dissolved in THF (100 mL, Aldrich 178810) (see FIG. 10). Potassium carbonate (1.08g, 7.8 mmol) was then added to the solution to neutralize any inorganic acidic products from previous step. The mixture was stirred for 10 minutes. Next, sulfanilate sodium salt (1.52 g, 7.8 mmol, Aldrich 251283) was added, and the mixture was heated to reflux (65° C., in air) overnight. The insoluble solid (excess potassium carbonate and sulfanilate sodium salt) was then filtered and discarded. The clear...
example 3
Preparation of the Sulfanilic Acid Liquid Crystal Monomer
[0090] The liquid crystal monomer may be synthesized by running the sodium polymerizable surfactant of Example 2 through an ion-exchanger resin. The sodium sulfanilate surfactant was synthesized by following steps 1 and 2 in Example 2.
[0091] The acidic ion-exchange column was prepared from AG 50W-X8 Resin (100 g, Bio-Rad, 143-5451) in THF. The resin was first stirred in a solution of HCl (200 mL, 6M) for 3 h. The first HCl solution was then removed, and the resin was continually stirred in a second solution of HCl (200 mL, 6M) for 3 h. The second HCl solution was then removed, and the resin was finally stirred in a third solution of HCl (200 mL, 6M) for 12 h. The slurry gel was then packed in a column (3 cm diameter), washed with excess water to remove HCl, and then washed with THF (150 mL) to completely remove water from the column. Next, the sodium salt solution in THF was passed through the acidic ion-exchange resin. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com