Enhanced ultrasound detection with temperature-dependent contrast agents
a technology of contrast agent and ultrasound detection, which is applied in the field of ultrasound detection and imaging, can solve the problems of inability to detect pathological changes on or near vascular surfaces, the temperature-dependent ultrasound velocity of perfluorocarbon liquid has not been suggested to have any applicability in ultrasound imaging systems, and the detection of pathological changes may be compromised, etc., to achieve enhanced contrast imaging, little or no detectable change in acoustic reflectivity, and sensitive measurement of ultrasound reflectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
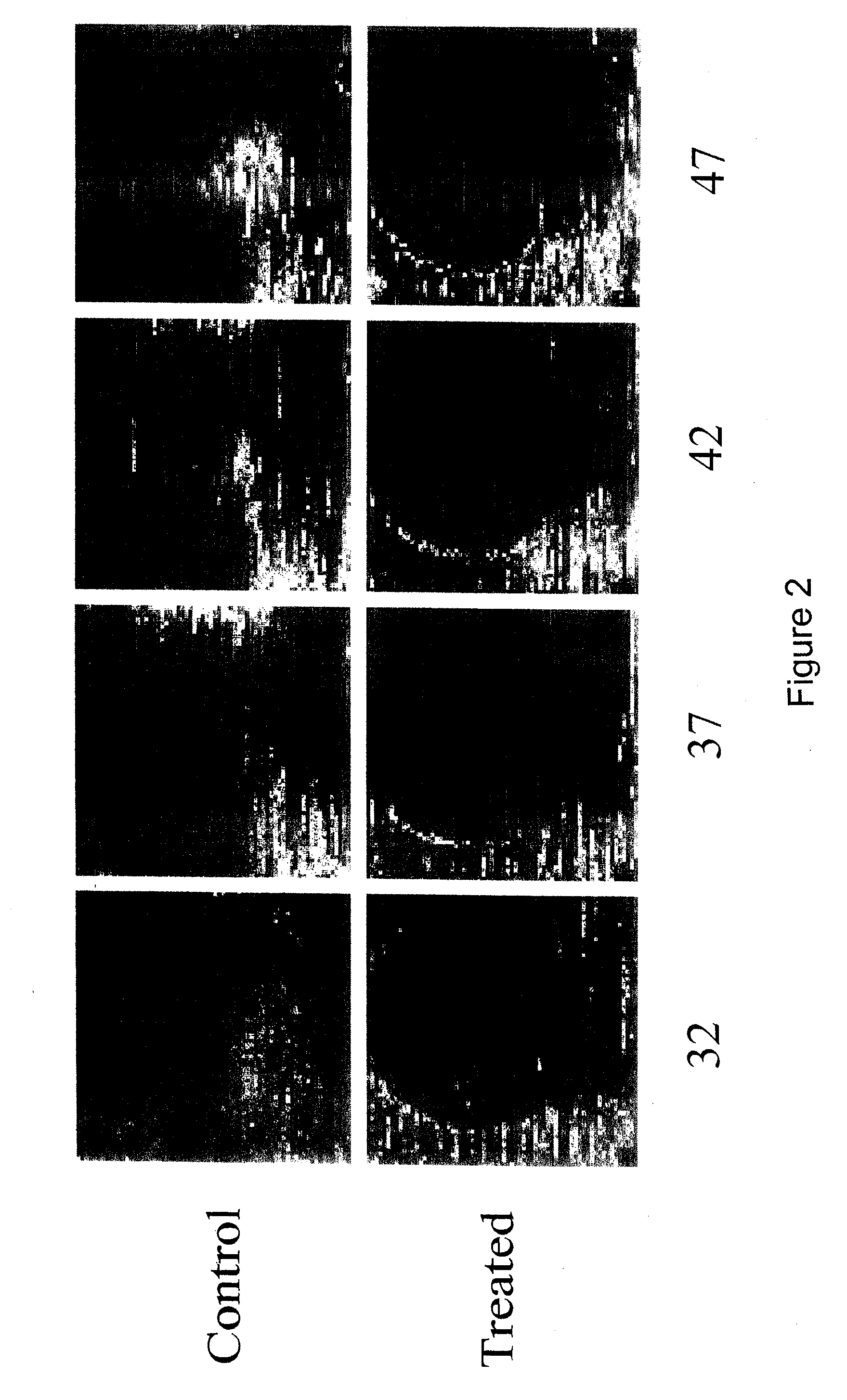
Image
Examples
example 1
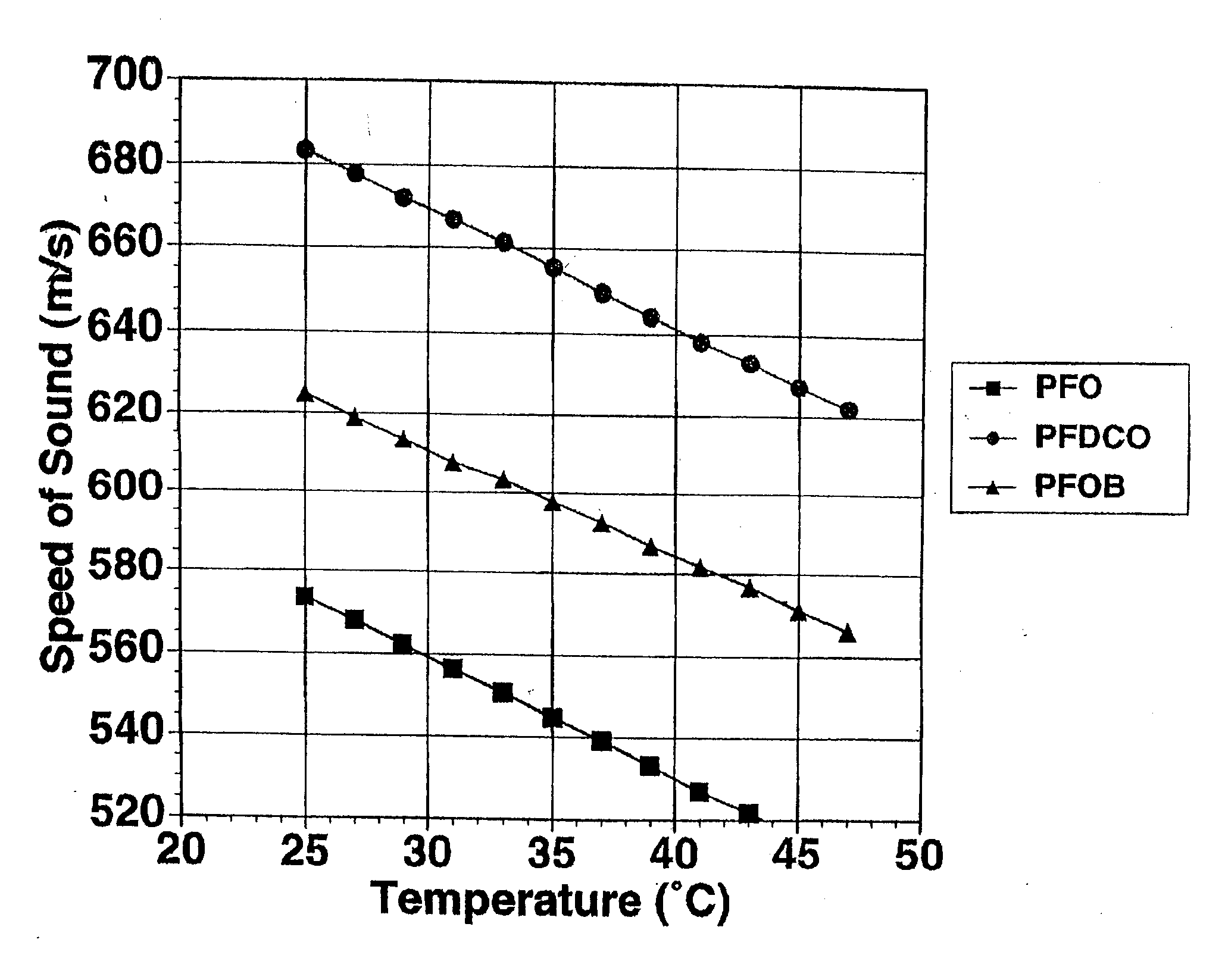
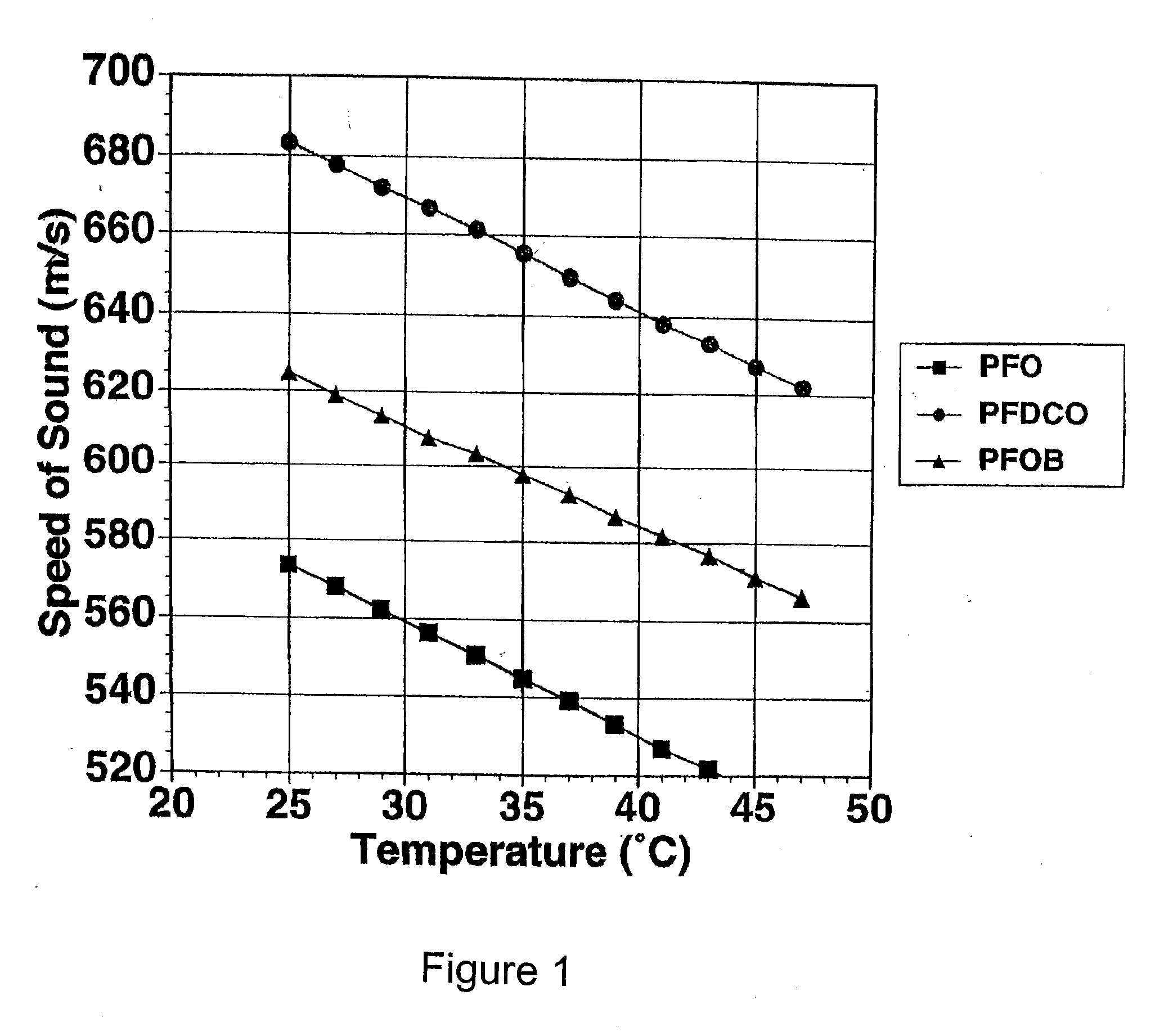
[0081] This example illustrates the measurement of temperature dependence of ultrasound velocity in perfluorooctane, perfluorodichlorooctane and perfluorooctylbromide.
[0082] Ultrasound velocities were determined using a 25 MHz, Panametrics V324 spherically focused transducer. Measurements were made for perfluorooctane, perfluorodichlorooctane or perfluorooctylbromide at discrete temperatures by placing 8 mL of fluorocarbon in a sealed, vertically mounted sample chamber in heated water bath. The back of the chamber consisted of a stainless steel reflector, which extended past the fully enclosed well to allow for water-path and sample-path measurements. The chamber was mounted so that the stainless steel reflector was perpendicular to the insonifying beam.
[0083] The times of flight from the transducer to front wall of the chamber and from the transducer to the stainless steel plate were determined for nine independent locations over the sample. The speeds of sound were then averaged...
example 2
[0085] This example illustrates the preparation of a biotinylated microemulsion for avidin-biotin targeting.
[0086] A biotinylated emulsion was prepared by incorporating biotinylated phosphatidylethanolamine into the outer lipid monolayer of a perfluorocarbon microemulsion. The microemulsion was prepared containing perfluorooctane (40% w / v, 3M), vegetable oil (2% w / v) a surfactant co-mixture (2.0%, w / v) and glycerin (1.7%, w / v) in water as follows. The surfactant co-mixture was prepared by dissolving 64 mole % lecithin (Pharmacia, Inc), 35 mole % cholesterol (Sigma Chemical Co.) and 1 mole % N-(6-(biotinoyl)amino) hexanoyl)-dipalmitoyl-L-alpha-phosphatidyl-ethanolamine, Pierce, Inc.) in chloroform. The chloroform-lipid mixture was evaporated under reduced pressure, dried in a 50° C. vacuum oven overnight and dispersed into water by sonication. The suspension was then transferred into a blender cup (Dynamics Corporation of America) with the fluorocarbon, vegetable oil, glycerin and d...
example 3
[0087] This example illustrates a method which can be used to prepare an emulsion in which the nanoparticles are conjugated with an F(ab) fragment.
[0088] Targeting of emulsions can be achieved by direct chemical conjugation of an antibody to the nanoparticle through a primer material incorporated into the lipid monolayer. The perfluorocarbon nanoparticle contrast agent is prepared as described in Example 1.
[0089] F(ab) fragments are generated and isolated using an immunopure F(ab) preparation kit (Pierce, Rockford, Ill.). Briefly, IgG is dialyzed into 20 mM phosphate / 10 mM EDTA buffer (pH 7.0), concentrated to 20 mg / ml and digested by immobilized papain. Solubilized F(ab) is purified from Fe fragments and undigested IgG protein using a protein A column. F(ab) fragments is purified from excess cysteine using a G25-150 column and deoxygenated phosphate buffer (pH 6.7). Fraction identity is confirmed by routine SDS-PAGE procedures.
[0090] F(ab) fractions are pooled and combined with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com