Compositions of Allosteric Hemoglobin Modifiers and Methods of Making the Same
a technology of allosteric hemoglobin and modifier, which is applied in the field of compositions of allosteric hemoglobin modifier compounds, can solve the problems of high exothermicity, inability to easily adapt to the environment, and difficulty in generating pharmaceutical grade compositions, so as to improve the sensitivity, improve the method sensitivity, and maintain precision and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
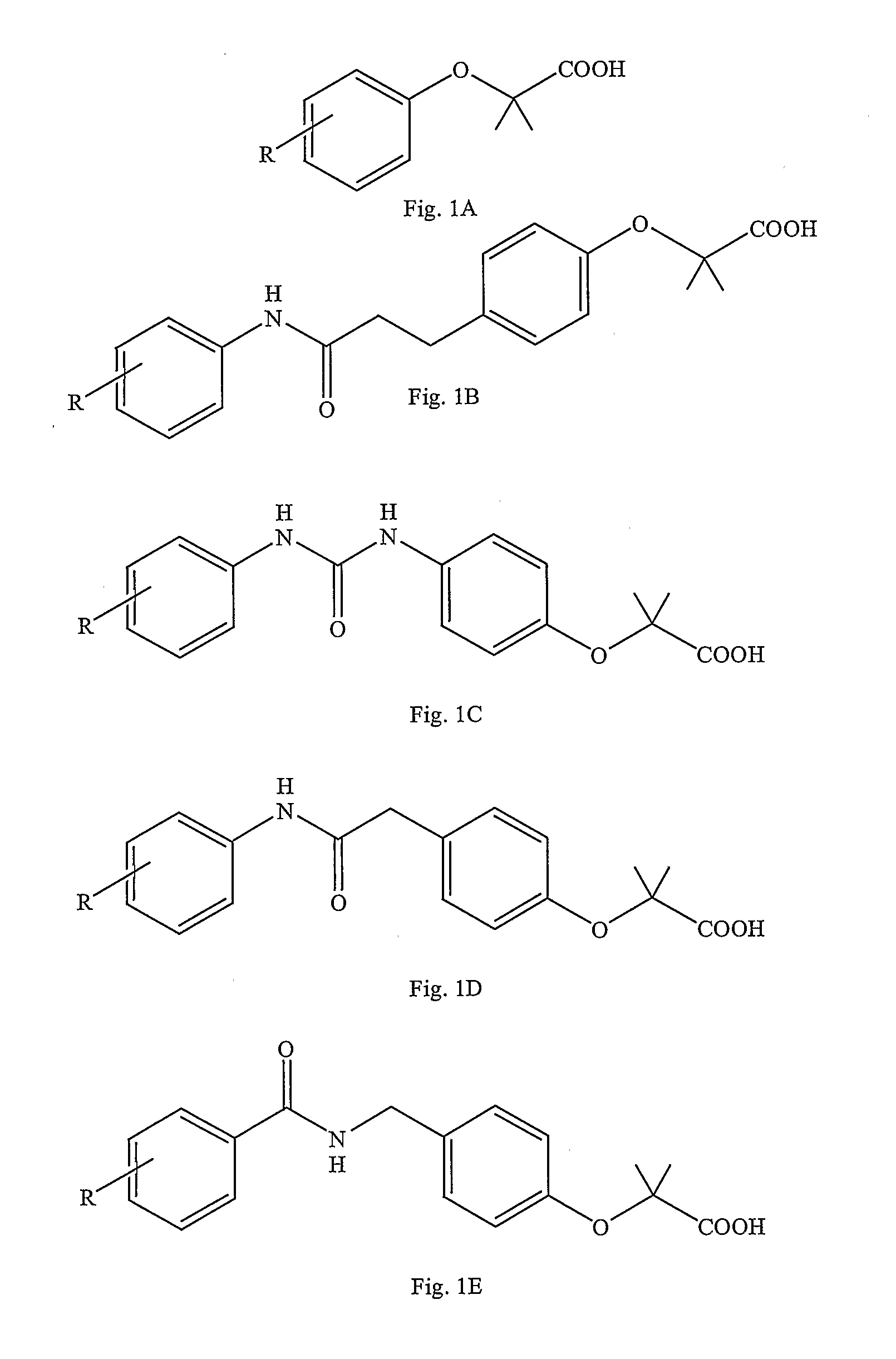
Preparation of 2-[4-((((3 5-dimethylphenyl)amino)carbonyl)methyl)phenoxy]-2-methyl propionic acid
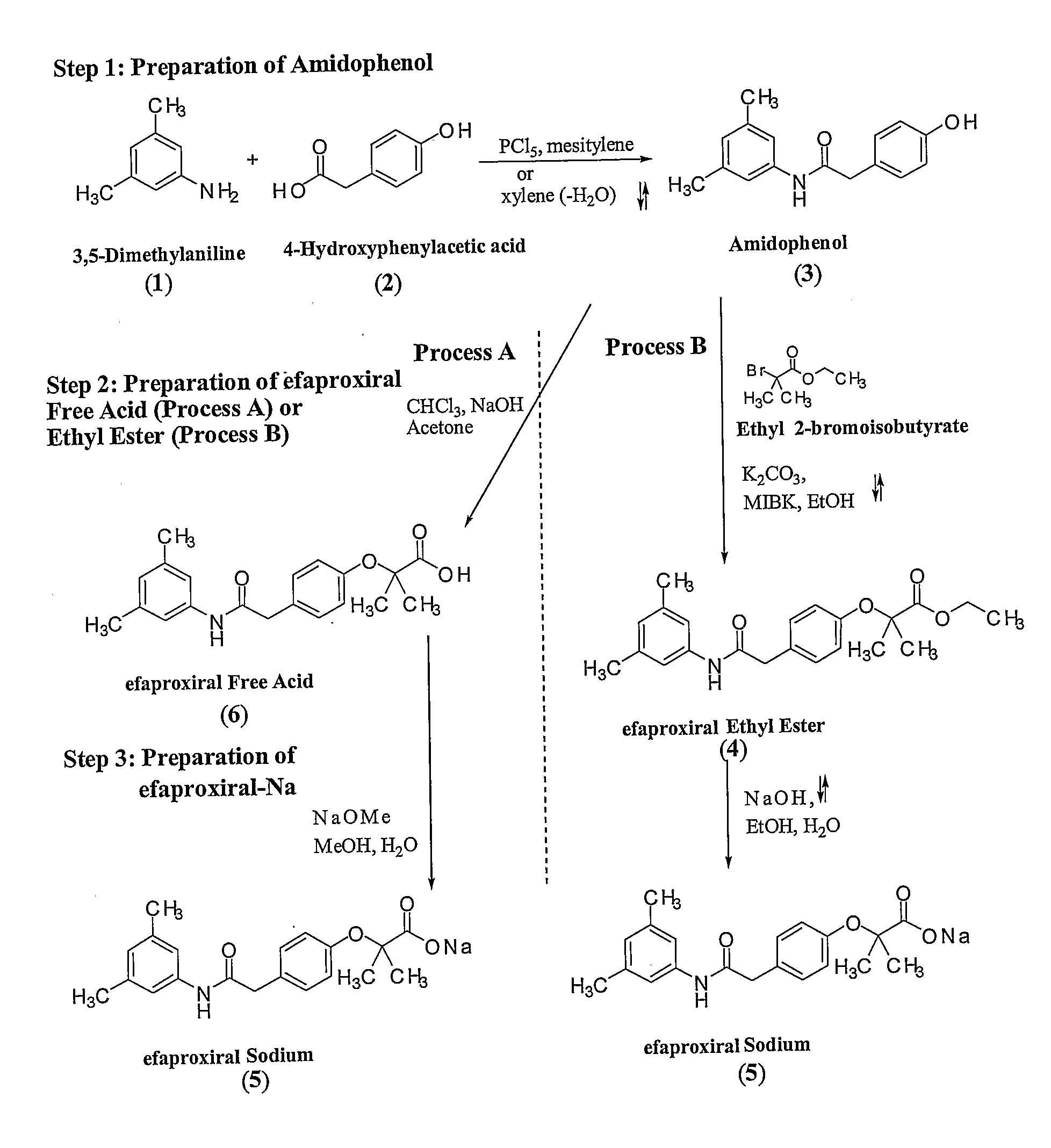
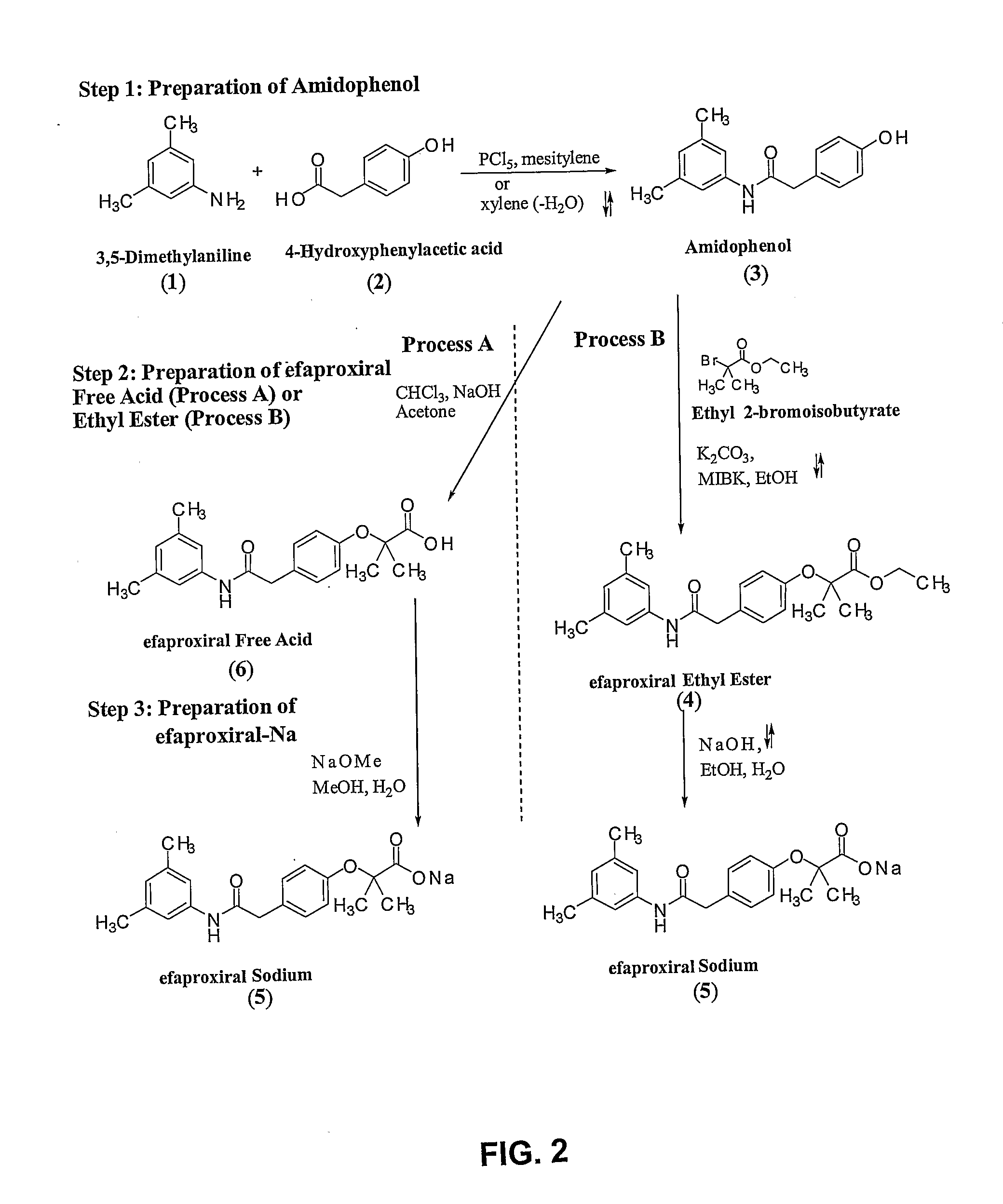
[0094]FIG. 3 illustrates a general five step reaction scheme for the preparation of 2-[4-((((3,5-dimethylphenyl)amino)carbonyl)methyl)phenoxy]-2-methyl propionic acid which is described in detail below.
Synthesis of Amidophenol (3)
[0095] With reference to FIG. 3, 4-hydroxyphenylacetic acid (2001 kg) (2) was added to xylene (760 L) with stirring. To this mixture, 3,5-xylidine (3,5-dimethyl aniline) (178 L) (1) was added. The reaction mixture was heated to reflux and water was removed azeotropically as the reaction proceeded. Upon completion, the reaction mixture was distilled to provide amidophenol (3), which solidified upon cooling. To recrystallize, ethanol (1180 L) and methyl isobutyl ketone (MIBK) (56 L) were added to the solid and the mixture was refluxed until dissolution. Upon dissolution water was added (70° C., 490 L) and mixture was stirred and cooled slowly over 6 hours to ab...
example 2
Purification of Efaproxiral Sodium (5) by Extraction with MIBK
[0098] Purified water (1658 L) was added to the product (5) (325 kg) obtained using the method described in Example 1. The mixture was distilled under vacuum at a maximum temperature of 50° C. until about 423 L of solvent was removed. Another 423 L of purified water was then added and the aqueous solution was extracted with MIBK (390 L, below 30° C.). The organic phase was discarded, the aqueous phase was extracted again with MIBK (228 L, below 30° C.) and the organic phase was discarded.
example 3
Purification of Efaproxiral Sodium (5) by Recrystallization with Acetone / Ethanol
[0099] The sodium salt of efaproxiral (5) synthesized as described in Examples 1 and 2 in the aqueous solution was concentrated under vacuum at a maximum temperature of 50° C. to the maximum extraction of solvent, after which absolute ethanol (406 L) was added to provide a mixture having a water content of between 5 and 5.4%. The mixture was then cooled to about 47° C., acetone (975 L) was added and the mixture was stirred while maintaining the temperature. After crystallization, the mixture was stirred for at least one hour, after which an equal volume of acetone was added. The mixture was then slowly cooled to a temperature of about 15° C. and stirred for at least 5 hours. The crystals were collected on a filter and washed with acetone (146 L).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com