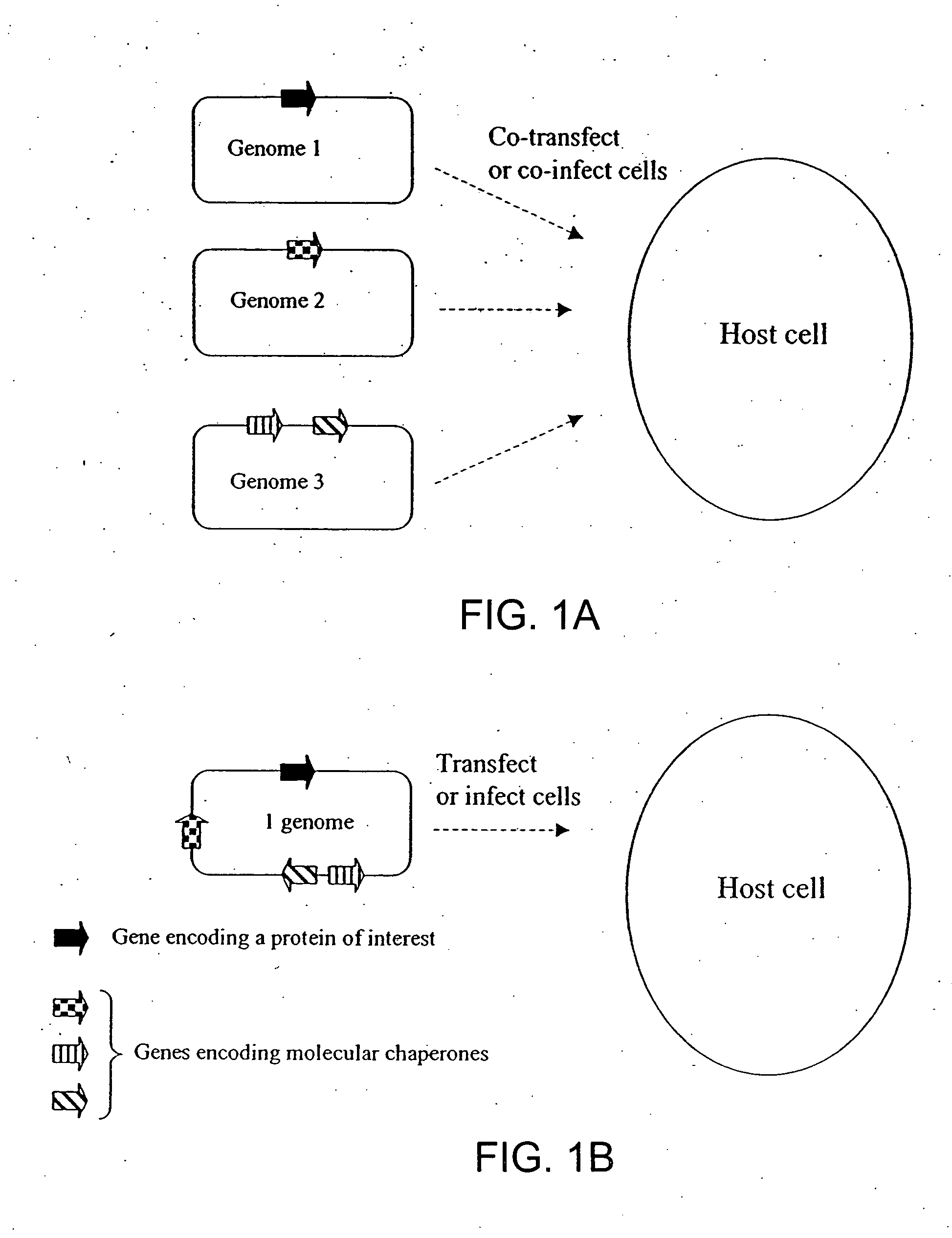
Chaperone expression genomes
a technology of genomes and chaperones, applied in the field of chaperone expression genomes, can solve the problem of inability to control the expression ratio of foreign proteins in individual cells, and achieve the effects of facilitating co-translocation, and improving the folding of a protein of interes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of an EGT Transfer Vector (AB-EGT)
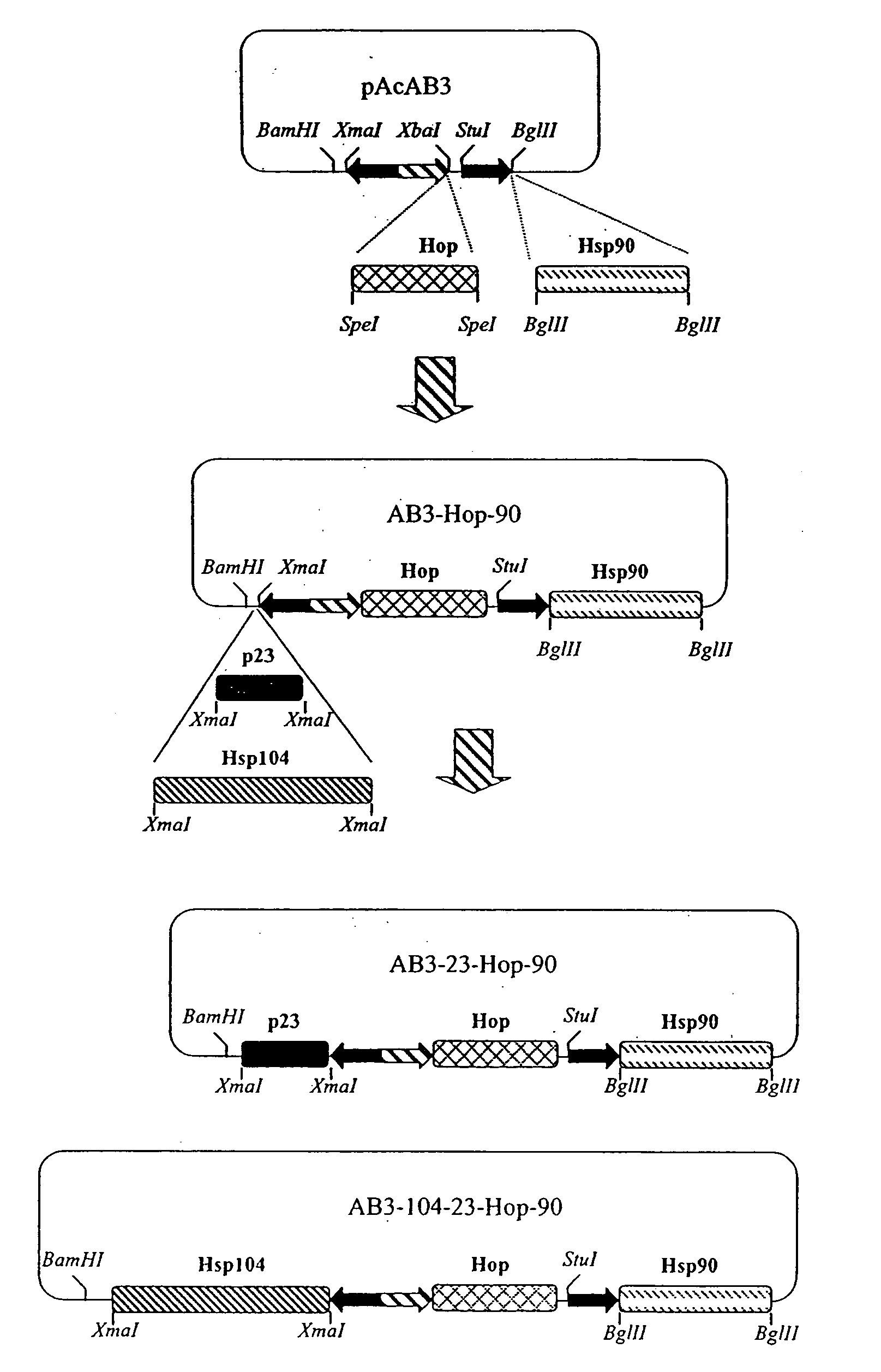
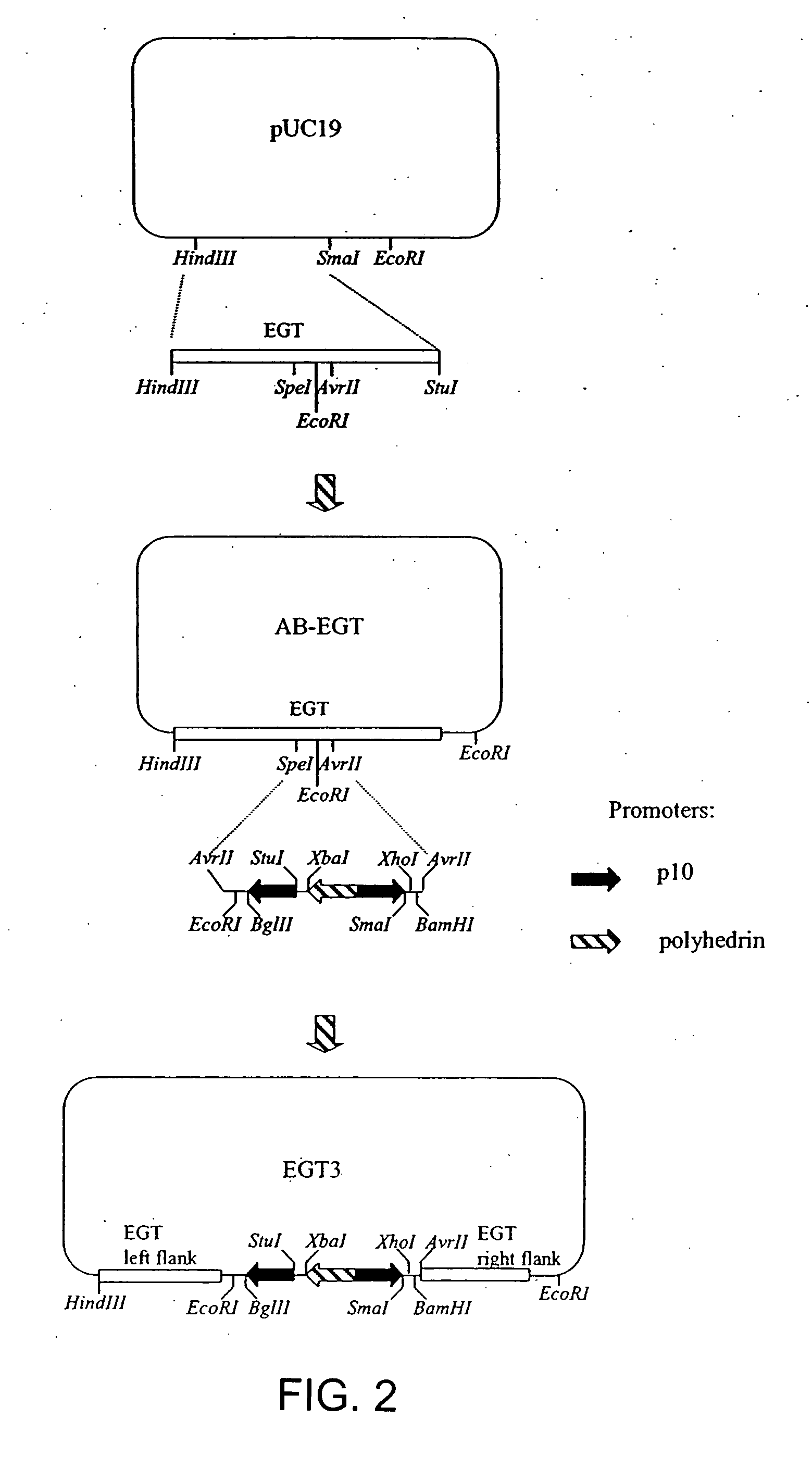
[0089] To construct EGT transfer vector according to present invention, a DNA fragment comprising EGT gene polynucleotide sequence is cloned into the pUC19 plasmid vector (Acc# X02514) cleaved with HindIII and SmaI restriction endonucleases (FIG. 2). The EGT gene can be obtained from AcNMPV DNA (Acc# NC—001623, Smith G. E. and Summers, M. D., U.S. Pat. No. 4,879,236, 1987) or any of its derivatives, for example BacPAK6 recombinant baculovirus vector DNA (Kitts P. et al., Biotechniques, 14: 810-817, 1993) or Bac-to-Bac, Bac-N-Blue, BaculoDirect vector DNA (Invitrogen, Carlsbad, Calif.). BacPAK6 DNA is available from several sources, for example Pharmingen (San Diego, Calif.), Clontech (Palo Alto, Calif.), Orbigen, (San Diego, Calif.). Alternatively, any other baculovirus DNA containing EGT gene can be used for this purpose. Primers, complementary to opposite strands of the baculovirus DNA are selected in order to provide for amplificati...
example 2
Multiple EGT Transfer Vector (EGT3)
[0091] Multiple expression vectors are desirable when there is a need to facilitate expression of several genes in the same vector. This example describes insertion of 3 promoters into AB-EGT vector in order to facilitate expression of up to 3 polynucleotide sequences of interest. Expression of a polynucleotide sequences of interest can be achieved using EGT promoter resident in the EGT vectors. However, employment of much stronger promoters, such as polyhedrin, p10 or a basic protein promoter is preferred (Bonning B. C. et al., J. Gen. Virol., 75 (Pt 7):1551-1556, 1994). If the same promoter is employed twice in the same cassette, it is preferred that its copies are facing in opposite directions, more preferably away from each other. This is done in order to avoid unstable direct polynucleotide sequence repeats and to minimize interference between transcription complexes moving along the DNA molecule.
[0092] A promoter cassette containing 3 promo...
example 3
EGT Transfer Vector with a Reporter Gene (EGT3-GFP)
[0095] This example describes insertion of a polynucleotide sequence of interest, which facilitates selection of recombinant baculoviruses obtained by recombination with a backbone plasmid transfer vector. More specifically, it describes insertion of such polynucleotide sequence into the EGT3 transfer vector, and more specifically, insertion of a gene encoding for a reporter protein, such as GFP (FIG. 3). A GFP gene, derived from jellyfish Aequeorea victoria, is amplified in PCR from a DNA containing such gene. Plasmid DNA containing this gene is available from Columbia University, New York or it can be purchased from commercial sources, for instance pGFP plasmid available from (Clontech, Palo Alto, Calif.). DNA containing GFP gene is amplified in PCR using following primers:
[SEQ ID NO: 5]5′-GCGCGGATCCAAAAAATGAGTAAAGGAGAAGAACTTTTCACTGG-3′[SEQ ID NO: 6]5′-GCGCGGATCCTCTTTGTATAGTTCATCCATGCCATGTG-3′
[0096] BamHI restriction endonuclea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| excitation wavelength | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com