Synthetic Compounds and Derivatives as Modulators of Smoking or Nicotine Ingestion and Lung Cancer
a technology which is applied in the field of synthetic compounds and derivatives as modulators of smoking or nicotine ingestion and lung cancer, can solve the problems of reducing the pharmacological activity reducing the success rate of current therapies to reduce smoking (e.g., nicotine patches), and many of the metabolites and constituents of tobacco and tobacco smoke are toxic, so as to reduce the risk of developing cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Chemical Synthesis of Substituted Tetrahydrofuran Nicotine Analogs
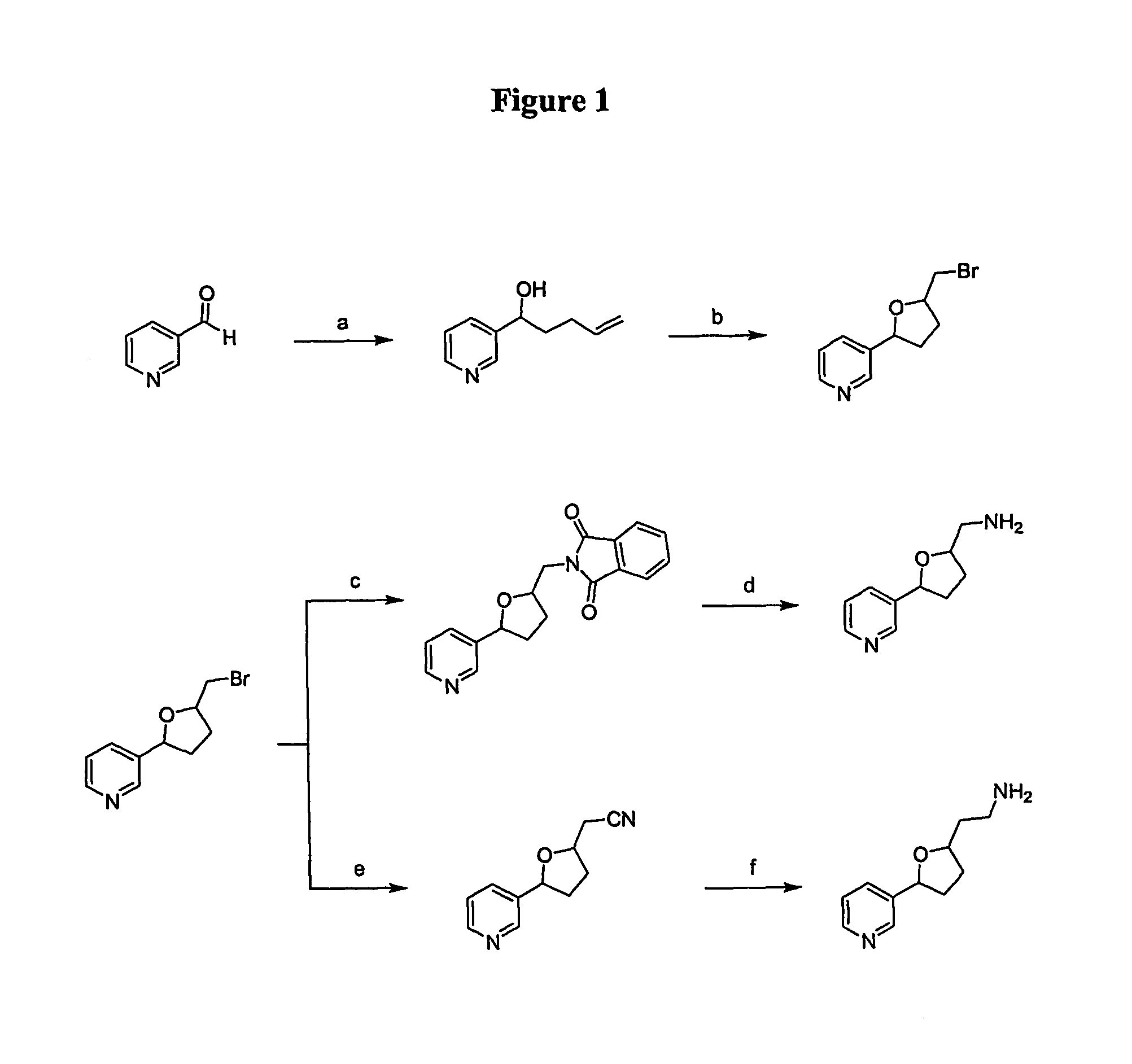
[0197]The synthesis of analogs of nicotine where the pyrrolidine ring was replaced with a substituted tetrahydrofuran ring system to mimic the parent compound were synthesized in a multistep sequence that provided the target compounds in overall acceptable yields. The synthesis is shown in FIG. 1. Generally, the bromide was derivatized with various functional groups to obtain the desired target compounds. The target compounds were fully characterized spectrally and subjected to biological evaluation. Purity was evaluated by HPLC analysis of the compounds.
example 2
Chemical Synthesis of Substituted Heteroaryl Analogs of Nicotine
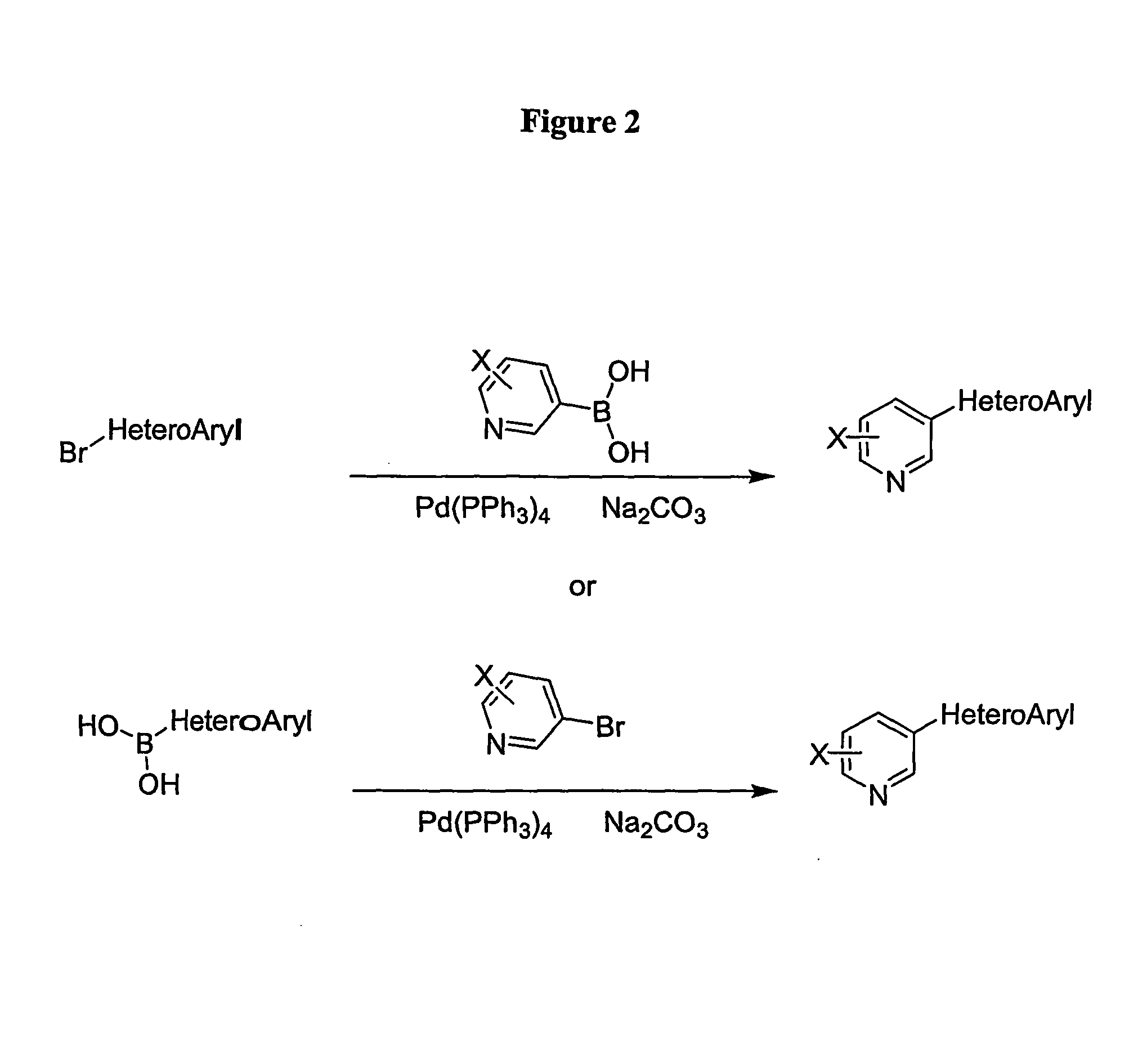
[0198]The synthesis of analogues of nicotine where the pyrrolidine ring was replaced with a heteroaryl group to mimic the parent compound is shown in FIG. 2. The appropriate aryl bromide was treated with tetrakis(triphenylphosphine)palladium(0), sodium carbonate as the base and appropriate boronic acid for 1 hour at elevated temperature. The products were obtained after workup and column chromatography in yields ranging from 40-95%. The products were characterized by NMR and mass spectrometry and subjected to biological evaluation.
example 3
Chemical Synthesis of Substituted Carbocyclic Analogs of Nicotine
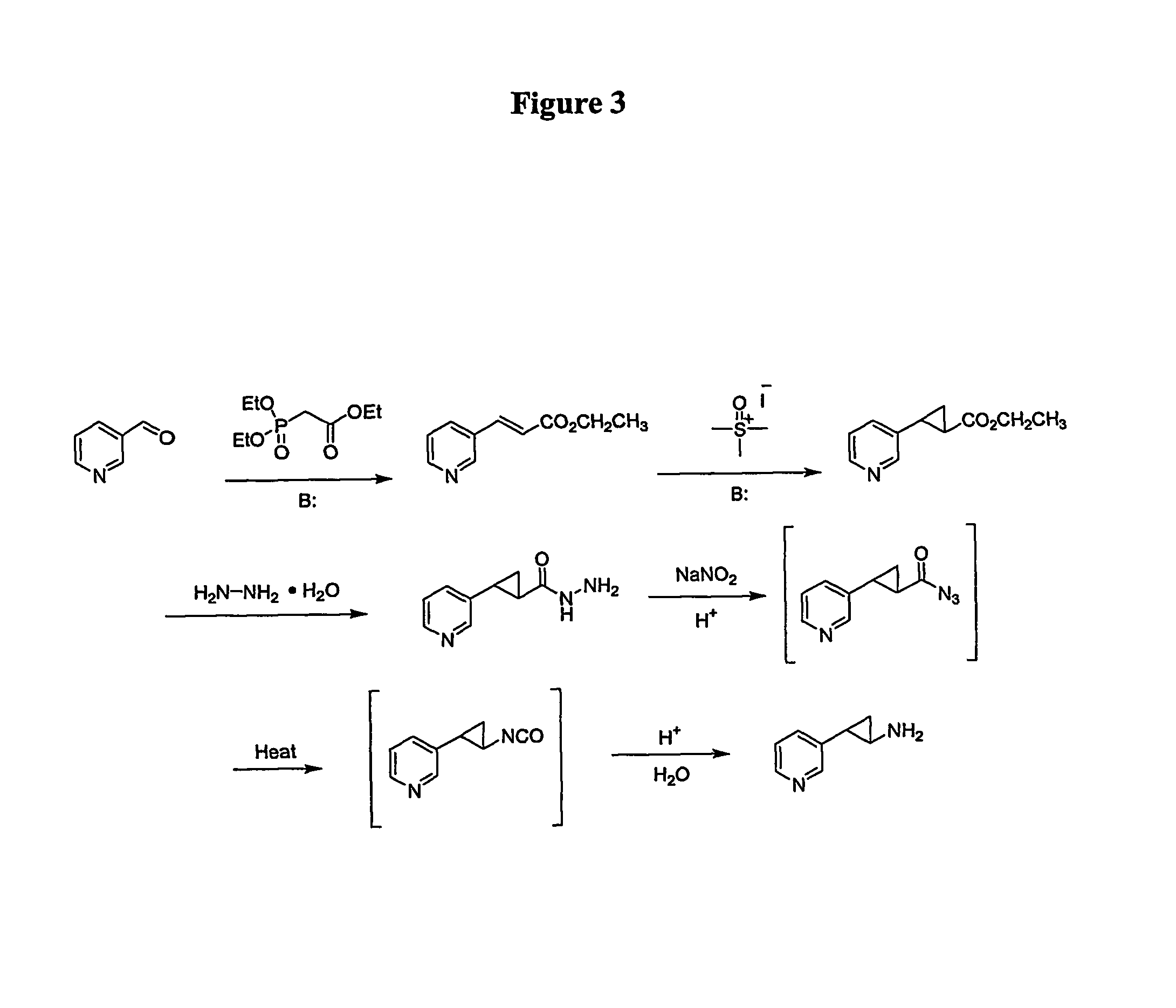
[0199]The synthesis of analogues of nicotine where the pyrrolidine ring was replaced with a substituted cyclopropyl ring system to mimic the parent compound is shown in FIG. 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| biological half-time | aaaaa | aaaaa |
| biological half-time | aaaaa | aaaaa |
| biological half-time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com