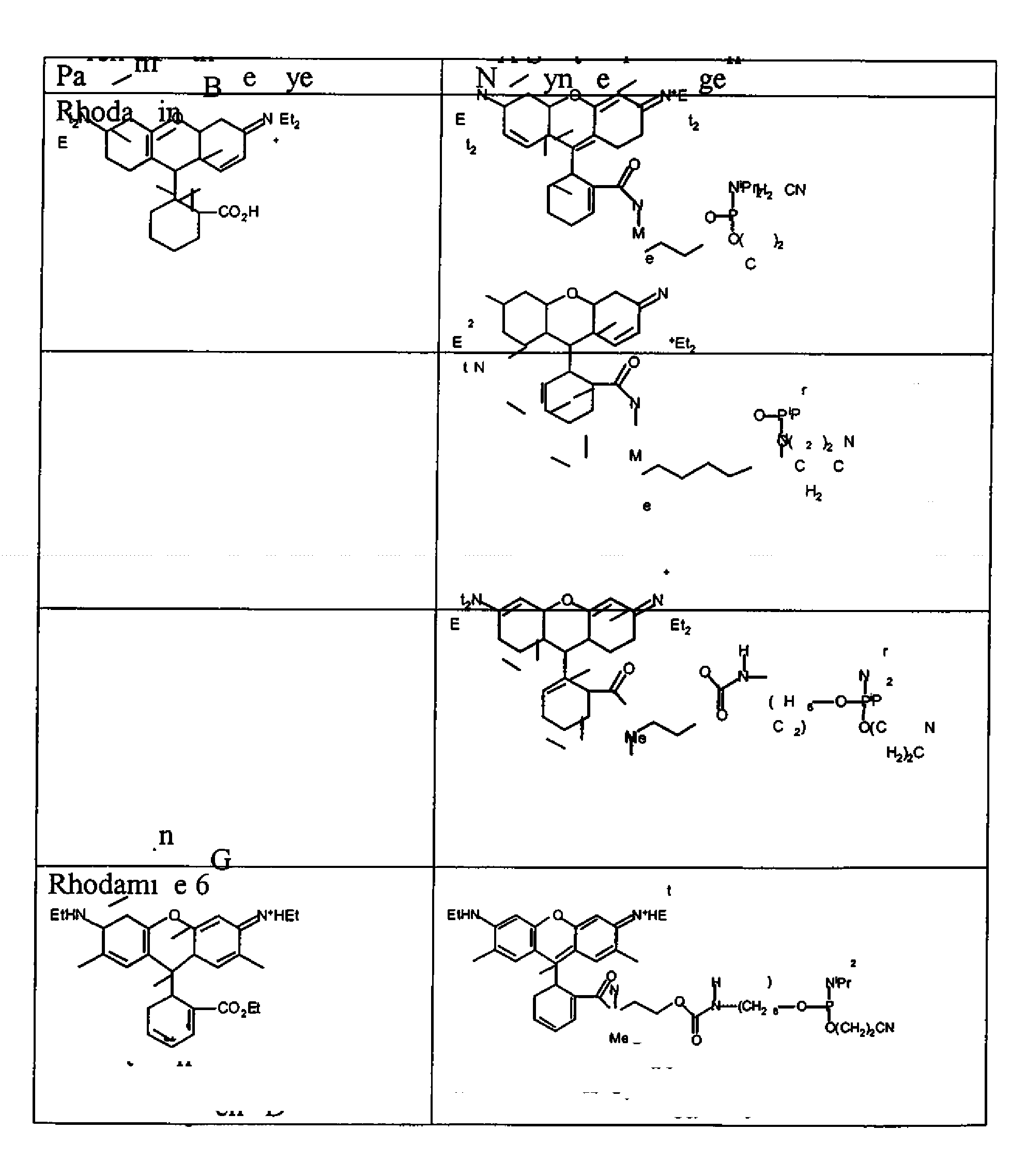
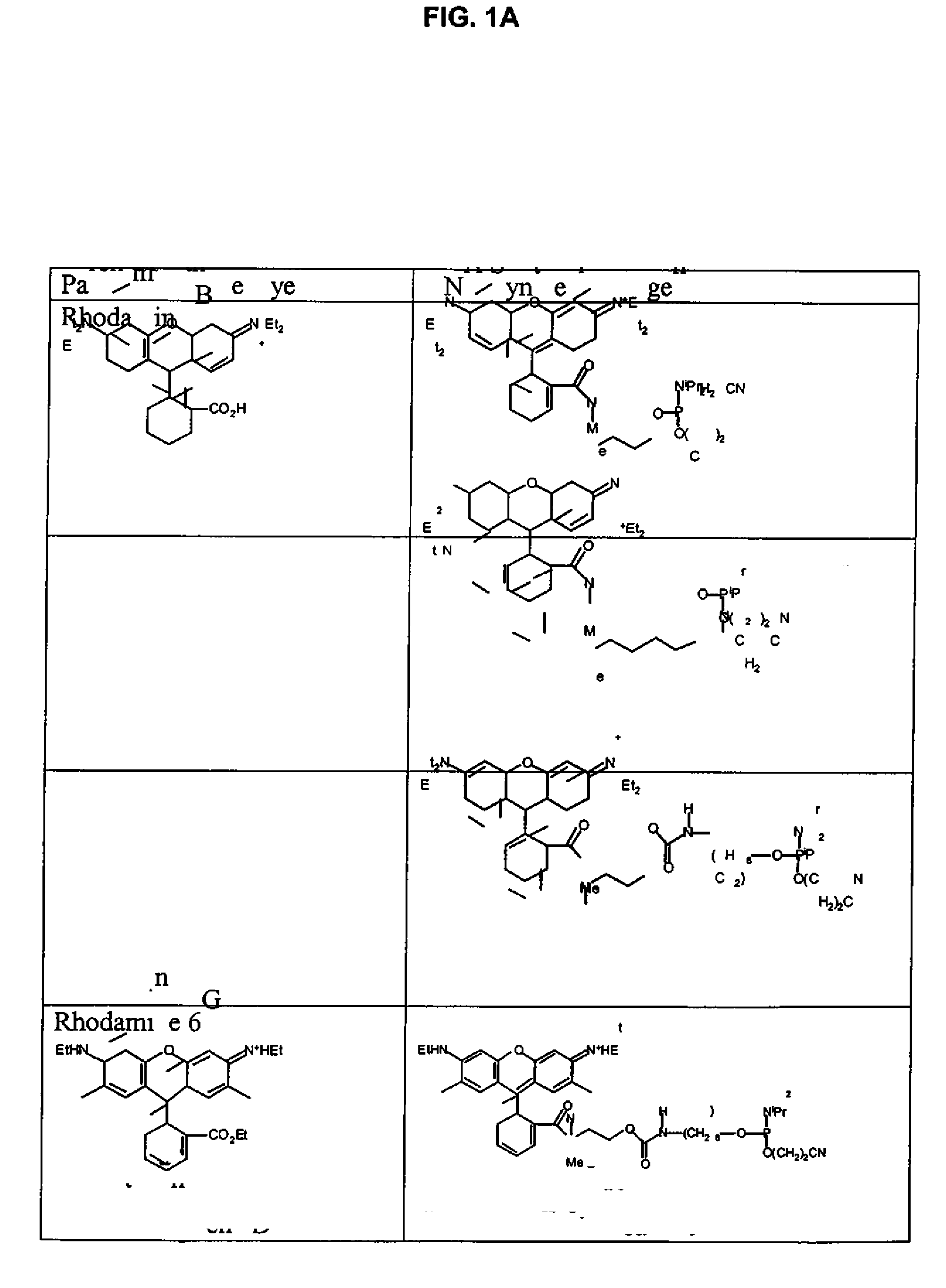
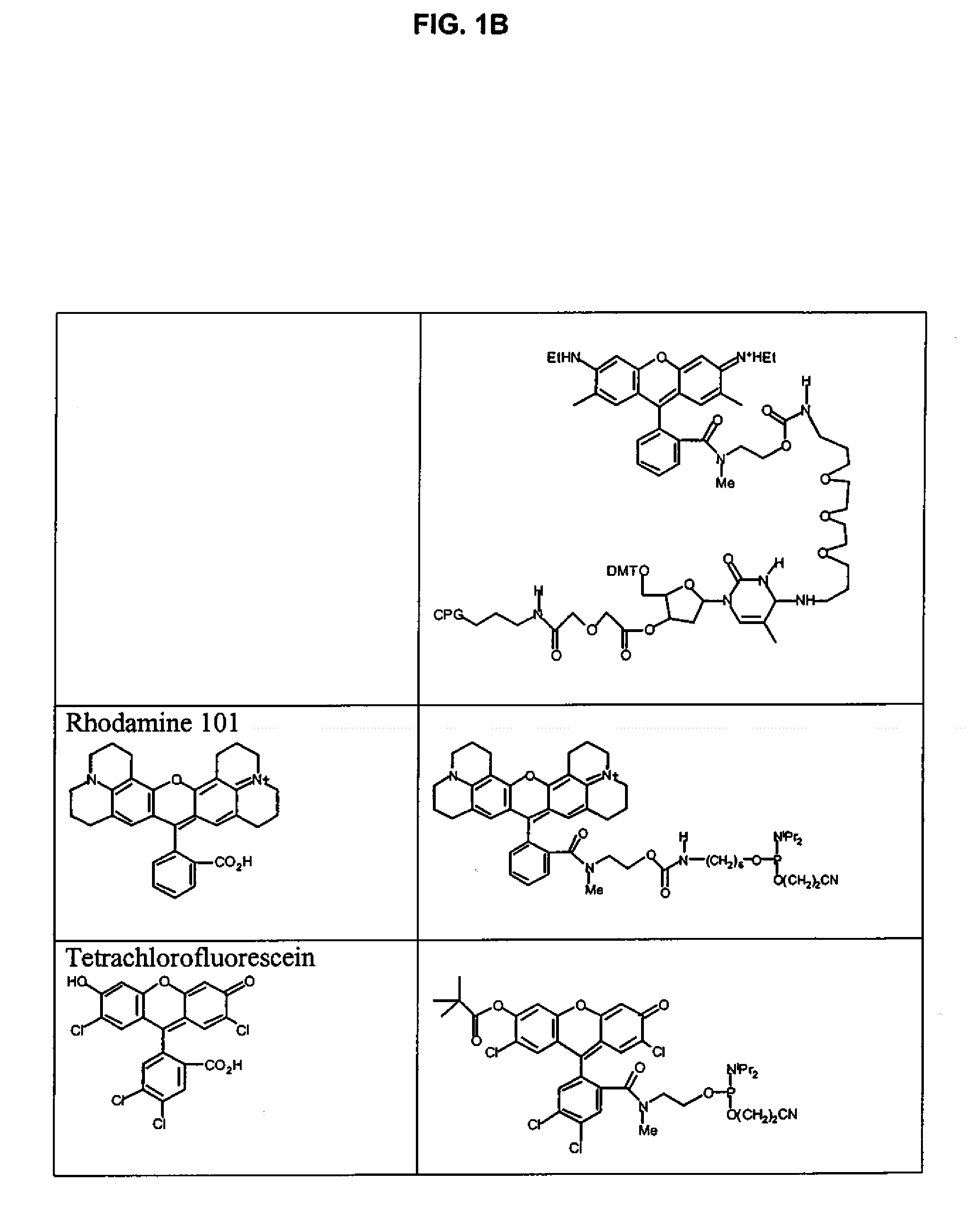
Xanthene dyes
a technology of xanthene dye and dye, which is applied in the direction of peptide sources, instruments, and organic compounds of the group 5/15 element, can solve the problems of displacing alternative methods, requiring sophisticated equipment and trained personnel, and expensive labels, and achieves the effect of being easily altered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1.1 Preparation of Rhodamine B acid chloride 1
[0266]
[0267]To a 500 mL round bottom flask were added 80% rhodamine B (6.0 g, 5.0 mmol) and phosphorus oxychloride (50 mL). The flask was fitted with a condenser and calcium sulfate drying tube. The reaction mixture was heated at reflux for 16 h and then cooled to room temperature. The volatile components were removed under high vacuum. Acetonitrile (100 mL) was added to dissolve the residue and was then removed by rotary evaporation and high vacuum. The solid material was again dissolved in acetonitrile (100 mL) and stripped to dryness to afford crude rhodamine B acid chloride.
1.2 Rhodamine B N-methylaminobutanol hexafluorophosphate 2
[0268]
[0269]Crude rhodamine B acid chloride (5.13 g, approx. 8.2 mmol) was dissolved in a mixture of DMF (20 mL) and acetonitrile (70 mL) and to this solution was added a solution of N-methylaminobutanol (3.0 g, 29.1 mmol) and triethylamine (7 mL) in acetonitrile (10 mL). The flask was fitted with a septum ...
example 2
2.1 Preparation of Rhodamine 6G acid 9
[0282]
[0283]Rhodamine 6G (17.3 g, 36.4 mmol) was dissolved in DMSO (320 mL) and 1N aqueous sodium hydroxide (80 mL) was added. The mixture was stirred for 16 h and then was neutralized by addition of 1N aqueous hydrochloric acid. The solid was filtered off and was dissolved in the minimum amount of methanol (approx. 500 mL). The solution was added to 1N aqueous hydrochloric acid (1200 mL) and then the methanol was boiled off. After cooling the solid was filtered off and was washed with water (2×30 mL). The solid was dried to afford the R6G acid (16 g, 97%).
2.2 Preparation of Rhodamine R6G succinimidyl ester 10
[0284]
[0285]To a 100 mL round bottom flask was added rhodamine R6G acid (6.63 g, 14.7 mmol), TSTU (5.25 g, 17.4 mmol), DMF (150 mL) and diisopropylethylamine (9 mL). The mixture was stirred at room temperature for 20 h. The solid was filtered off, was washed with DMF (2×4 mL) and acetonitrile (2×5 mL), and was dried to afford rhodamine R6G ...
example 3
3.1 Preparation of 19 from Rhodamine 101
[0296]
[0297]Compound 19 and it precursors 16-18 were prepared in a manner analogous to corresponding compounds of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescent | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com