Methods and compositions for determination of glycated proteins
a technology of glycated proteins and compositions, applied in the direction of instruments, peptides, enzymology, etc., can solve problems such as the disfunction of target molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Glycated Serum Protein Enzymatic Assay Kit
[0099]Intended Use. The exemplary assay kit is for determination of glycated serum proteins (fructosamine) in human serum. Fructosamine is formed due to a non-enzymatic Maillard reaction between glucose and amino acid residues of proteins. In diabetic patients, elevated blood glucose levels correlate with increased fructosamine formation. Fructosamine is a medium term indicator of diabetic control (2-3 weeks).
[0100]Assay Principle. The exemplary enzymatic assay for glycated serum proteins (GSP) uses Proteinase K to digest GSP into low molecular weight glycated protein fragments (GPF), and uses Diazyme's specific Fructosaminase™, a microorganism originated amadoriase to catalyze the oxidative degradation of Amadori product GPF to yield PF or amino acids, glucosone and H2O2. The H2O2 released is measured by a colorimetric Trinder end-point reaction. The absorbance at 550 nm is proportional to the concentration of glycated serum proteins (GSP)....
example 2
Glycated Serum Protein Assay Precision and Linearity
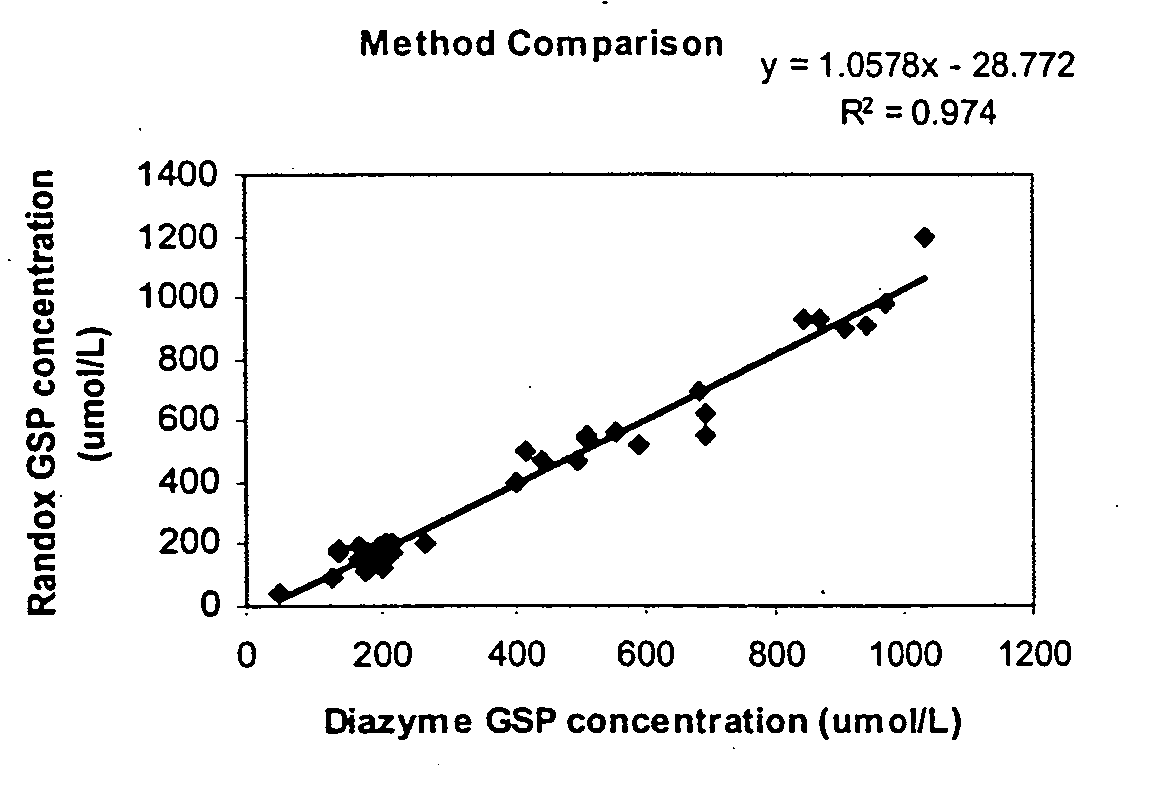
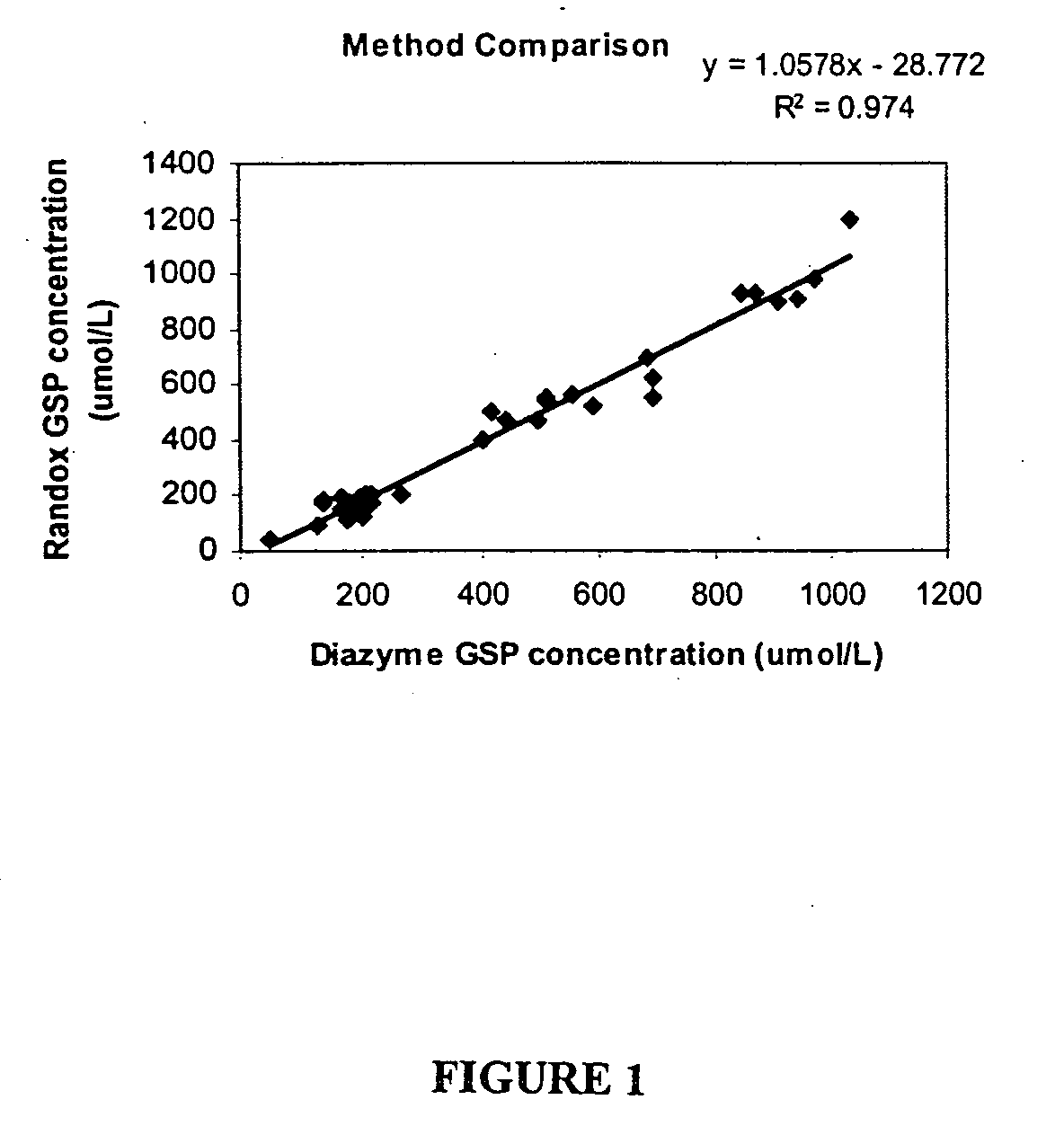
[0132]Method Comparison
[0133]Determined by running 2 replicates of a set of random samples using both Diazyme GSP kit and Randox Fructosamine kit in one run. The analytical performance characteristics determined by Diazyme GSP kit were comparable to those observed with Randox Fructosamine kit when assays were performed under the conditions as described in the Example 1 (See also FIG. 1).
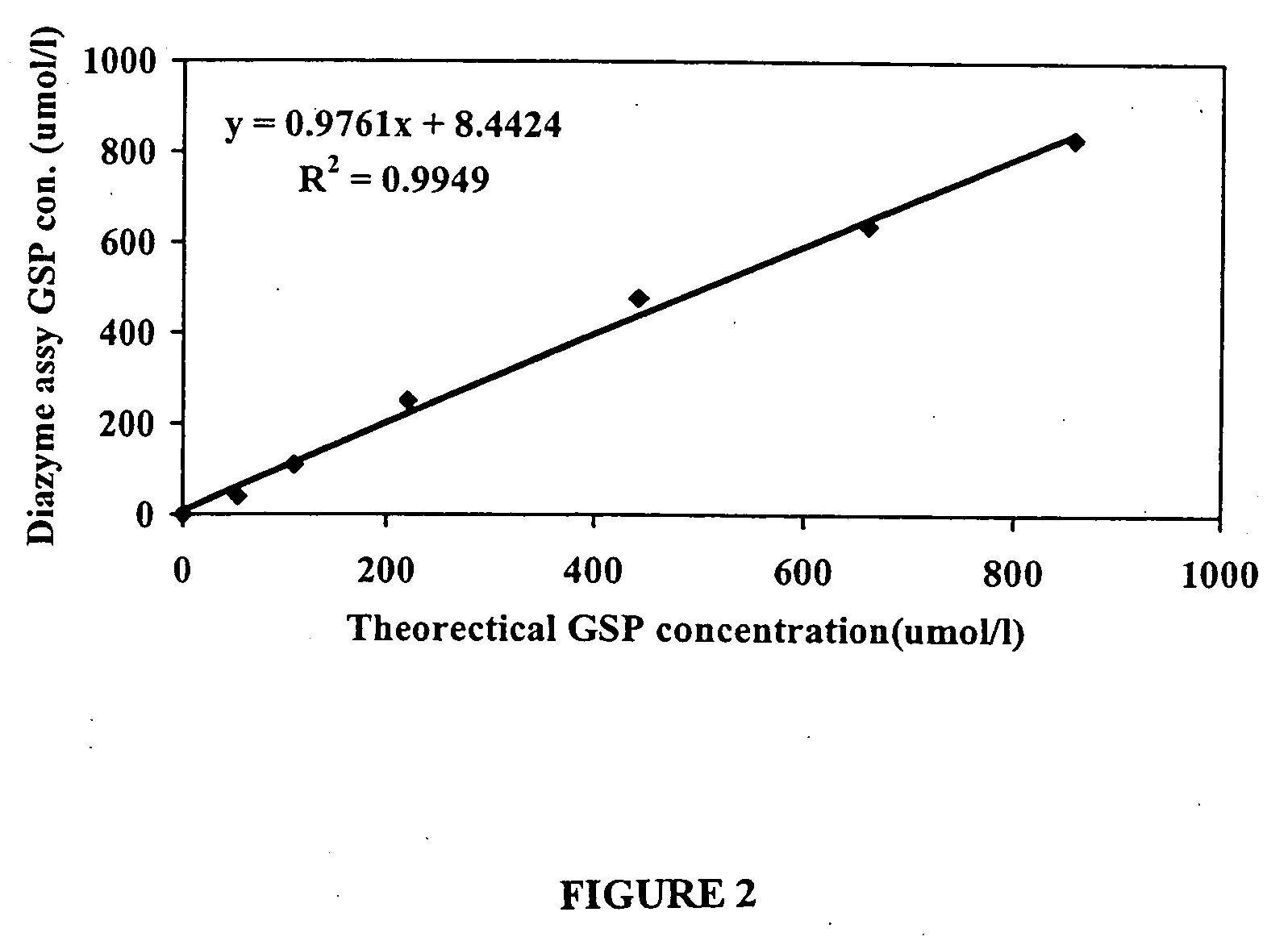
[0134]Assay Linearity
[0135]Determined by running 2 replicates of a set of series diluted serum samples in one run. The assay is linear from 40-856 umole / L (See FIG. 2).
[0136]Interference
[0137]Determined by running 3 replicates each of a control sample in the absence and presence of various potential interference substances at indicated concentrations (See the following Table 5).
TABLE 5Interference analysisInterfering substanceInterfering substancesconcentration% InterferenceAscorbic Acid 4 mg / dL−0.3Bilirubin 2 mg / dL−0.6Glucose1200 mg / dL −0.6Hemoglob...
example 3
Assay for Glycated Hemoglobin HbA1c
[0138]A. Glycated Valine Measurement:
[0139]1. Mix 10 ul 170 mM Glycated Valine (G-Valine) (This value was assumed all valine was converted to G-Valine in the cooking procedure.) with 300 ul R1 (80 mMCHES, 30 mmMOPS, 0.9% BRIJ). This mixture serves as sample stock solution (GVR1).
[0140]2. Set spectrometer wavelength at 726 nm, temperature at 37° C. Pipette 150 ul R2 (30 mMMES, 1 mMCaCl2, 2 mMWST-3, 1570 U / ml Proteinase K) to a cuvette, add 0 ul, 2.5 ul, 5 ul, 10 ul, 15 ul, 20 ul above GVR1 respectively for dose response, make up the sample volume to 20 ul with H2O in R1, incubate for 5 min, get the first O.D. reading, then add 30 ul R3 (0.08 mMDA-64, 240 mMTris, 180 U / ml HRP, 20 U / ml FAOD), incubate for 3 min, get the second O.D. reading. Calculate the O.D. difference between these two readings, using 20 ul H2O in R1 as control. FIGS. 3 and 4 show the dose-dependent reaction with fructosyl-valine.
[0141]B. Glycated Hemoglobin Measurement:
[0142]1. Mix...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com