Monoclonal antibody capable of antagonizing and inhibiting bonding of programmed death-1 receptor (PD-1) and ligand thereof as well as encoding sequence and use of monoclonal antibody
A programmed death and PD-1 technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody mimic/scaffold, anti-animal/human immunoglobulin, etc., can solve adverse events Reactions, high security risks and uncertainties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
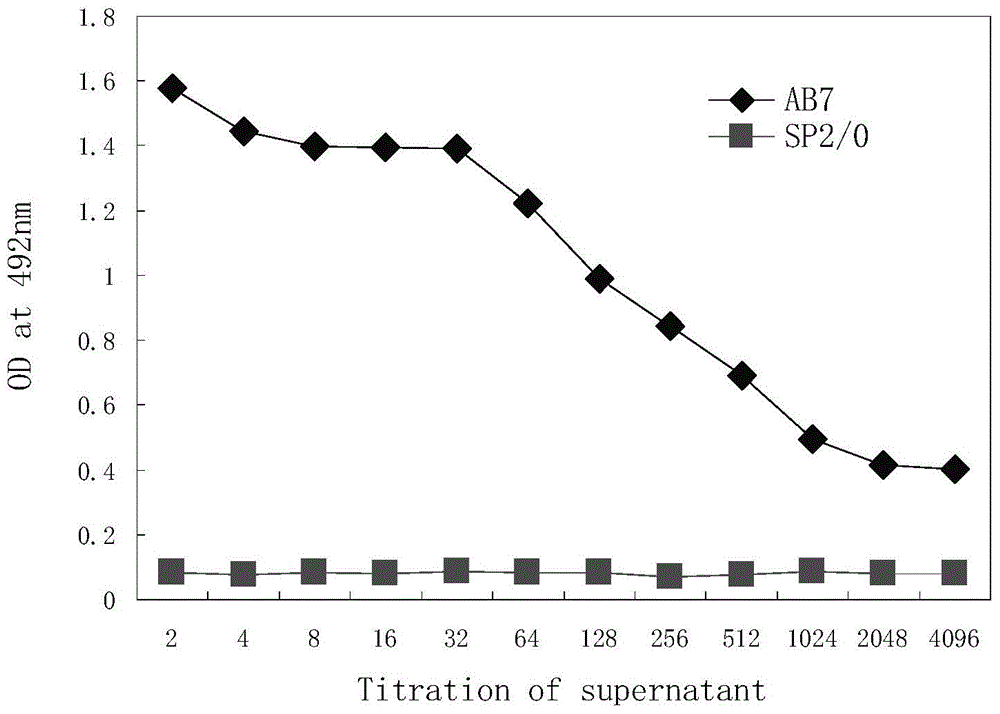
[0056] Example 1: Establishment and screening identification of mouse hybridoma cell lines secreting anti-PD-1 antibody
[0057] Step 1. Obtaining recombinant human PD-1 protein (immunization antigen) and animal immunization
[0058] In the embodiment of the present invention, the human PD-1 protein used for immunization is the recombinant human PD-1 extracellular protein expressed by mammals (product of Beijing Yiqiao Shenzhou Company). After the recombinant human PD-1 protein was mixed with complete Freund's adjuvant (product of Sigma, USA), Balb / c mice were injected subcutaneously at multiple points (100 μl / mouse, 5-10 μg PD-1 protein in total). 2-3 weeks after the first immunization, the mice were injected subcutaneously with a mixture containing PD-1 protein and Freund's incomplete adjuvant (product of Sigma, USA). After 2-3 booster immunizations, a small amount of mouse serum was taken , using the 96-plate coated with human PD-1 protein to detect the titer of anti-PD-1 an...
Embodiment 2
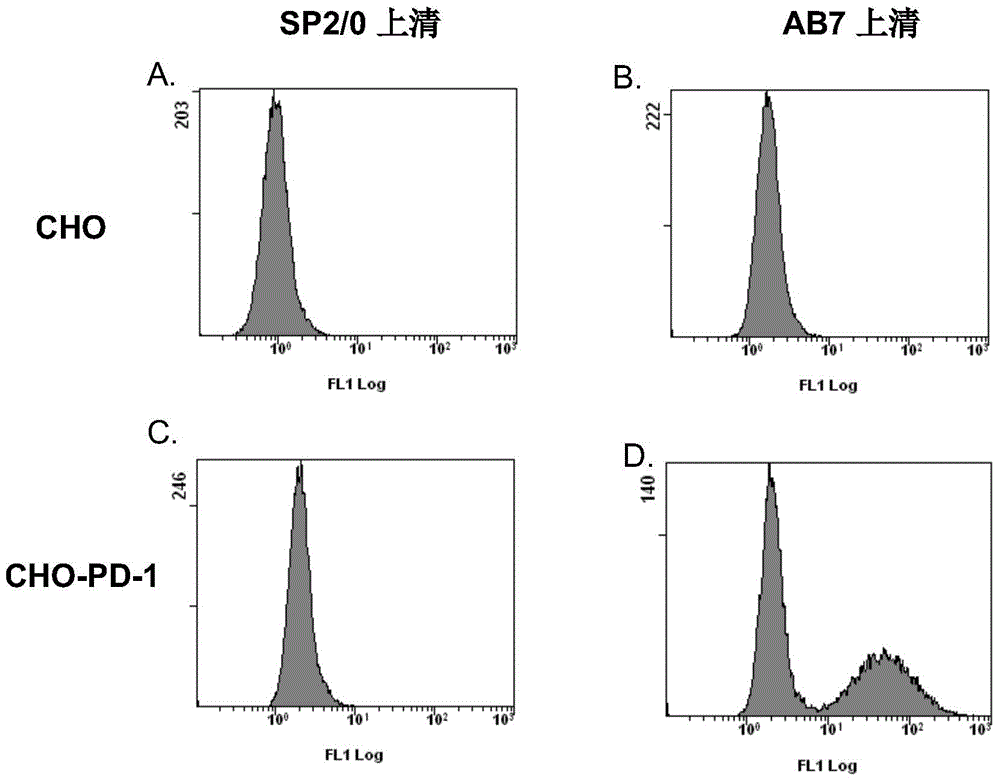
[0065] Example 2 Flow cytometry detection and analysis of the binding of murine AB7 monoclonal antibody to CHO cells transfected with human PD-1 gene
[0066] In this example, the AB7 antibody was used as the primary antibody, and FITC fluorescently labeled goat anti-mouse IgG was used as the secondary antibody, and flow cytometry was used to detect and analyze the binding of the AB7 antibody to CHO cells transfected with the human PD-1 gene. To this end, total mRNA was first extracted from human peripheral blood lymphocytes (PBL), and then amplified in vitro by Reverse transcription-polymerase chain reaction (RT-PCR) to obtain the full-length protein encoding PD-1 (including extracellular and intracellular region) cDNA fragment. The primers used for RT-PCR are PD-1 forward primers: ATTAAGCTTGAGCAGTGGAGAAGGCGGCA, Seq ID No: 11 , PD-1 reverse primer: AATTGGATCCCTCCTGAGGAAATGGGCTGA, Seq ID No: 12 ). The cDNA fragment amplified by the pair of primers covers the entire coding...
Embodiment 3
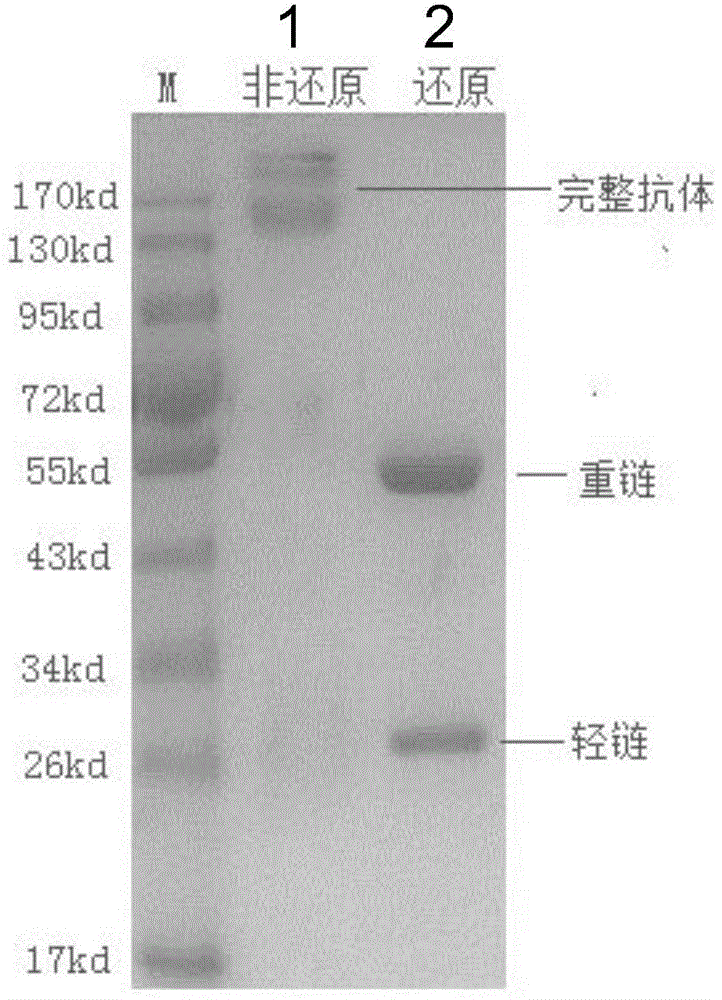
[0068] Example 3. In vitro preparation and purification of mouse-derived anti-human PD-1 monoclonal antibody (AB7)
[0069] In this example, the mouse anti-human PD-1 monoclonal antibody (AB7) protein was obtained by separation and purification by affinity chromatography.
[0070] Its purification steps are as follows:
[0071] After the AB7 hybridoma cells were amplified, they were inoculated in 200-500ml of serum-free 1640 medium, cultured at 37°C for 5 days, then the culture supernatant was collected, filtered through a 0.45μm filter membrane, and loaded onto Protein G-Sepharose Fast Flow (purchased from General Electric GE Company) affinity chromatography column; the chromatography column was first rinsed with PBS solution to remove impurity proteins, and then the adsorbed AB7 antibody was eluted with low pH (2.7-3.0) glycine (0.1M) solution protein. The eluate was adjusted to pH 7.0 with 1mol / L Tris (pH8.5-9.0), and then dialyzed against 10 times the volume of PBS solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com