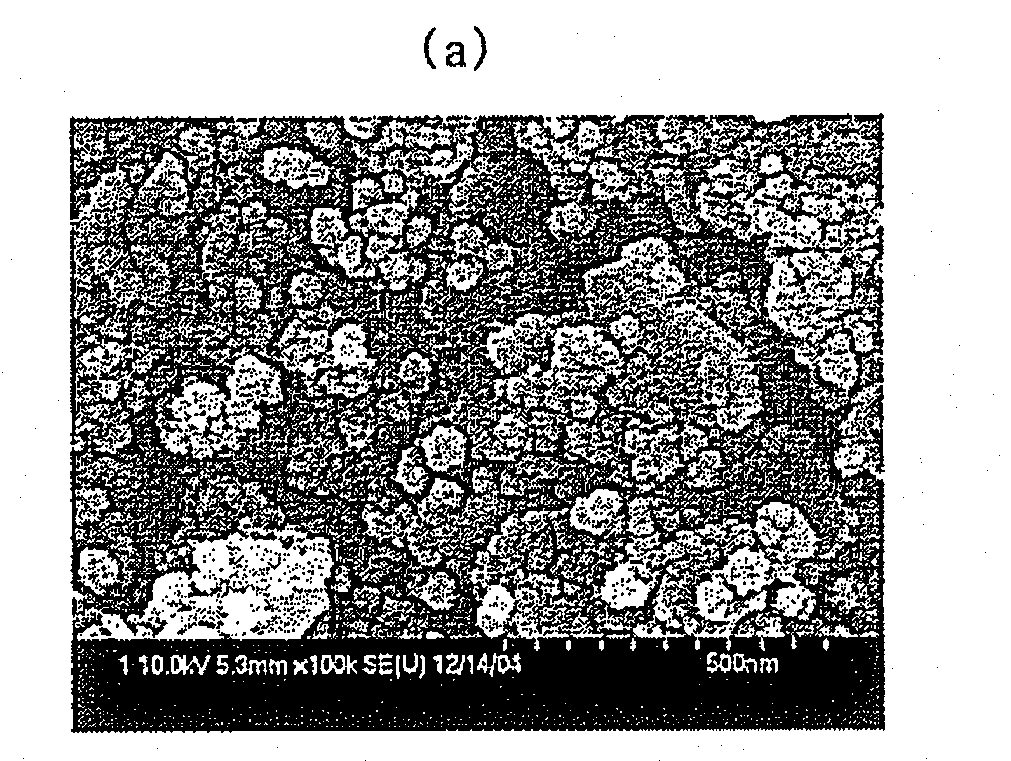
Metal Oxide with High Thermal Stability and Preparing Method Thereof
a technology of metal oxide and thermal stability, which is applied in the direction of catalyst activation/preparation, metal/metal-oxide/metal-hydroxide catalyst, etc., can solve the problems of reducing specific surface area, and inevitably exposed to high temperatures of three-way catalysts for the purification of exhaust gas of vehicles. , to achieve the effect of high specific surface area, high crystallinity and superior thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
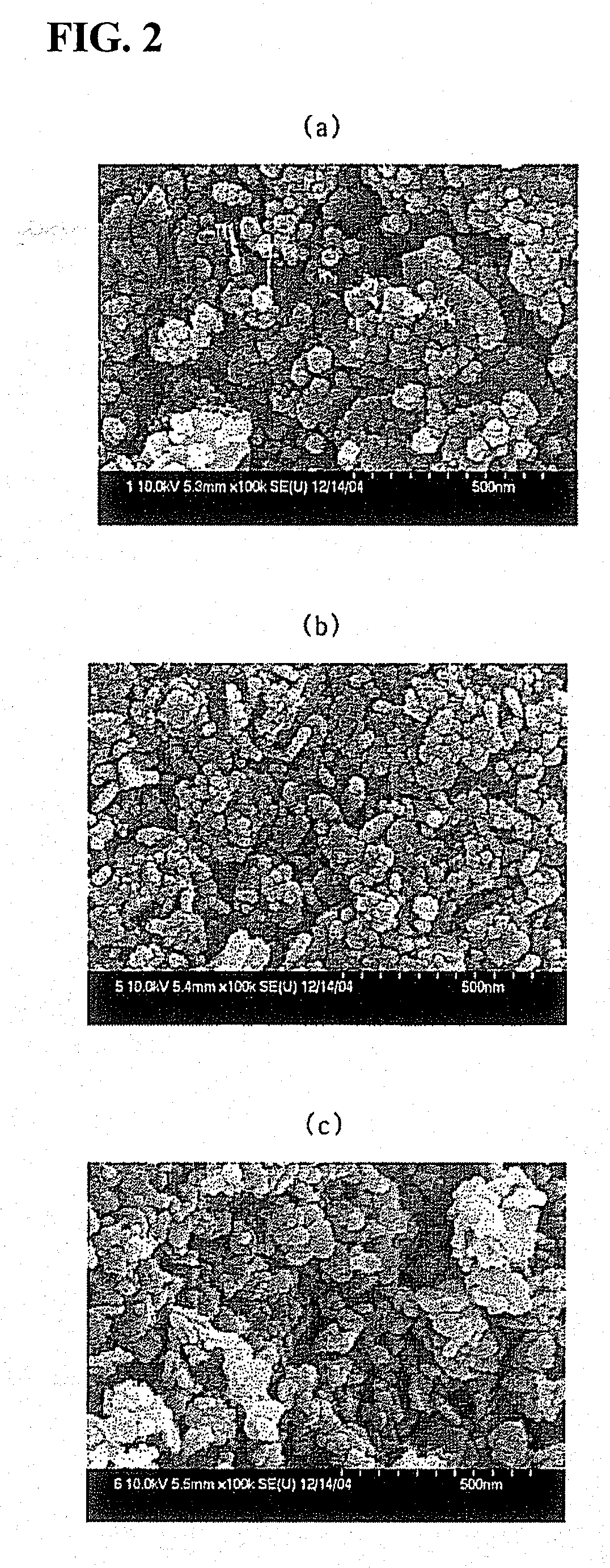
[0037]An aqueous mixture solution, comprising 5.81 wt % of zirconyl nitrate [30 wt % aqueous solution as ZrO2], 6.17 wt % of cerium nitrate [Ce(NO3)3.6H2O], and 9.02 wt % of aluminum nitrate [Al(NO3)3.9H2O], was pumped at a rate of 8 g per min through a tube having an outer diameter of ¼ inch and pressurized at 250 bar. 16.34 wt % of ammonia water [28 wt % NH3] was pumped at a rate of 8 g per min through a tube having an outer diameter of ¼ inch and pressurized at 250 bar. The pressurized aqueous mixture solution, comprising zirconyl nitrate, cerium nitrate, and aluminum nitrate, and the pressurized ammonia water were pumped into a tube-shaped continuous line mixer to thus be instantly mixed, and then allowed to precipitate for a residence time of about 30 sec. Further, deionized water was pumped at a rate of 96 g per min through a tube having an outer diameter of ¼ inch, preheated to 550° C., and pressurized at 250 bar. Subsequently, the deionized water thus preheated and the preci...
example 2
[0038]An aqueous mixture solution, comprising 2.43 wt % of zirconyl nitrate, 2.58 wt % of cerium nitrate, 0.37 wt % of lanthanum nitrate [La(NO3)3.6H2O], and 15.62 wt % of aluminum nitrate, was pumped at a rate of 8 g per min through a tube having an outer diameter of ¼ inch, and pressurized at 250 bar. 16.88 wt % of ammonia water was pumped at a rate of 8 g per min through a tube having an outer diameter of ¼ inch and pressurized at 250 bar. The pressurized aqueous mixture solution, comprising zirconyl nitrate, cerium nitrate, lanthanum nitrate, and aluminum nitrate, and the pressurized ammonia water were pumped into a tube-shaped continuous line mixer to thus be instantly mixed, and then allowed to precipitate for a residence time of about 30 sec. Further, deionized water was pumped at a rate of 96 g per min through a tube having an outer diameter of ¼ inch, preheated to 550° C., and pressurized at 250 bar. Subsequently, the preheated deionized water and the precipitate resulting ...
example 3
[0039]An aqueous mixture solution, comprising 2.10 wt % of zirconyl nitrate, 0.56 wt % of cerium nitrate, and 18.34 wt % of aluminum nitrate, was pumped at a rate of 8 g per min through a tube having an outer diameter of ¼ inch, and was pressurized at 250 bar. 17.17 wt % of ammonia water was pumped at a rate of 8 g per min through a tube having an outer diameter of ¼ inch and pressurized at 250 bar. The pressurized aqueous mixture solution, comprising zirconyl nitrate, cerium nitrate, and aluminum nitrate, and the pressurized ammonia water were pumped into a tube-shaped continuous line mixer to thus be instantly mixed, and were then allowed to precipitate for a residence time of about 30 sec. Further, deionized water was pumped at a rate of 96 g per min through a tube having an outer diameter of ¼ inch, preheated to 550° C., and pressurized at 250 bar. Subsequently, the preheated deionized water and the precipitate resulting from the line mixer, which were in a state of being pressu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com