Oral Compositions and Route of Administration for the Delivery of a Thylakoid Extract
a technology of thylakoid extract and oral composition, which is applied in the directions of magnoliophyta medical ingredients, plant ingredients, biocide, etc., can solve the problem that no data have confirmed the potential use of thylakoid extract as an oral anti-oxidative, and achieve the effect of preventing oxidative damage to components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
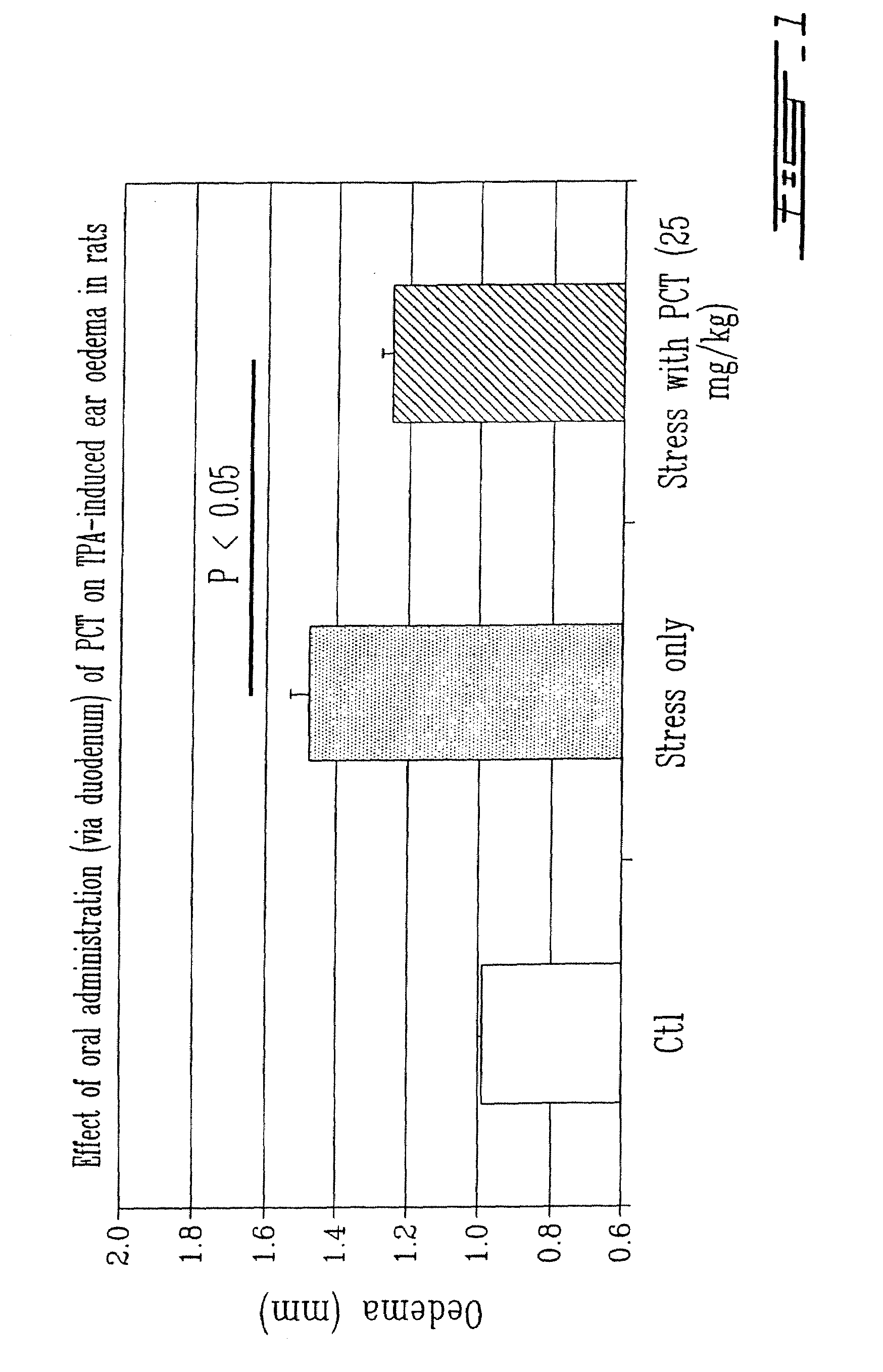
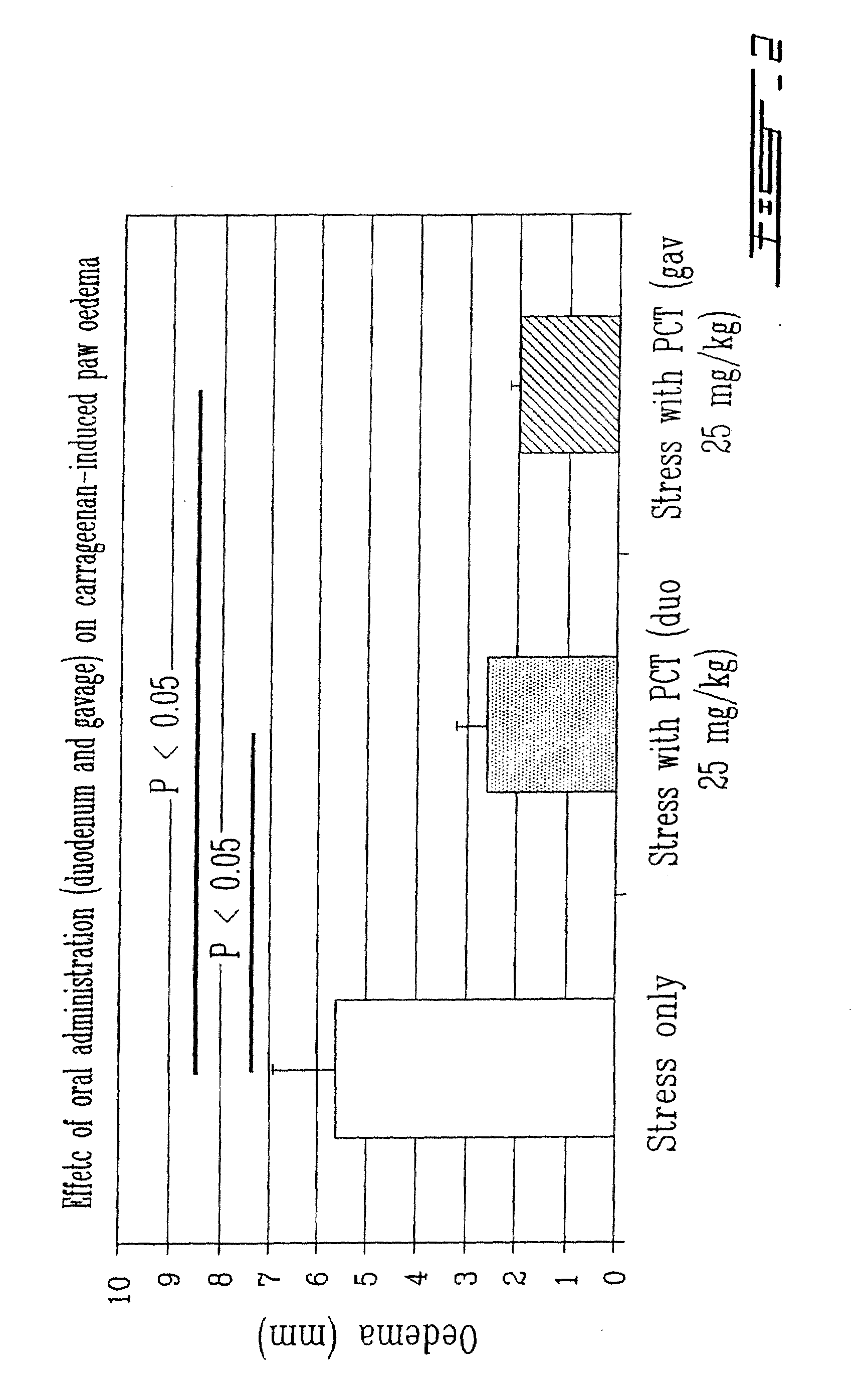
The Thylakoids are Active as Enteral and Oral Compounds
Methodology
Animals
[0031]Male Wistar rats (180-200 g) were used in the experiments. The animals were purchased from Charles River Canada (St-Constant, Qc, Canada). The animals were housed in an environmentally (t=25° C.) and air humidity (60%) controlled room with a 12 h light-dark cycle, kept on a standard laboratory diet and drinking water ad libitum. The experiments were approved by the ethical committee of TransBIOTech (Levis, Qc, Canada).
Reagents
[0032]12-O tetradecanoyl phorbol 13-acetate (TPA, P-8139) and carrageenan (C-1138) were purchased from Sigma Chemical Co. (St-Louis, Mo., USA).
Preparation of the Thylakoid Extract
[0033]The thylakoid extract was obtained from spinach leaves (Spinacia oleacea) as described in International patent publication WO 01 / 49305, the whole content of which is incorporated herein by reference. The thylakoids integrity was evaluated by spectrophotometry (Beckman DU 640) (Lichtenthale 1987) and fl...
example 2
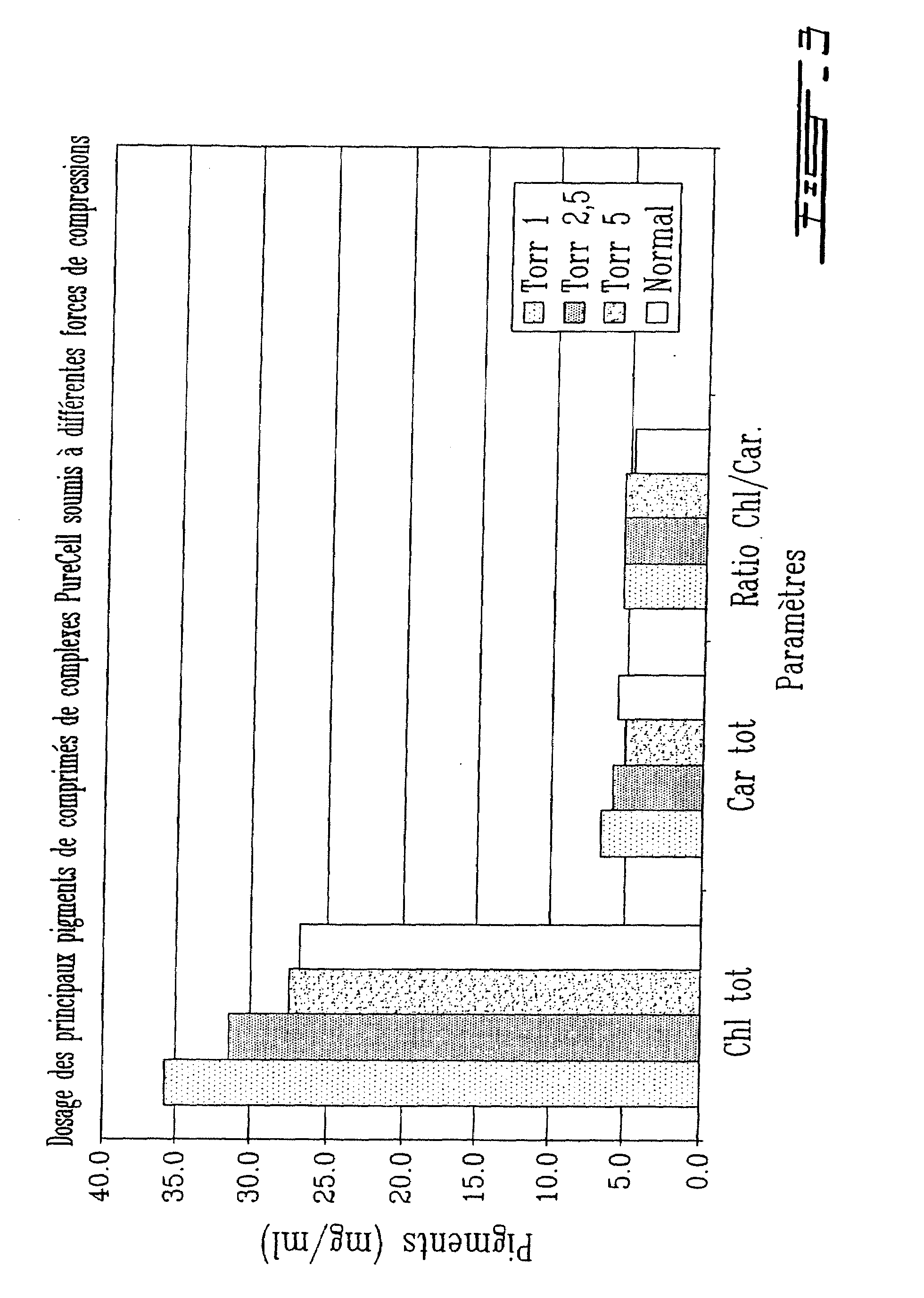
The Thylakoid Extract can be Formulated as a Product for Oral Use
Materials and Methods
Materials
[0043]Three commercially available polymers were used for this study sodium alginate, carboxymethyl cellulose low viscosity (CMC1) and carboxymethyl cellulose high viscosity (CMC2). The complex PCT was given by PureCell Technologies inc.
PCT Stability to Compression
[0044]First of all, PureCell Technologies inc. PTC was compressed as such, with any excipient, in order to evaluate the capacity of PCT to preserve its biological activity, following compression. Tablets of 200 mg made from PCT only were obtained by dry compression at 1, 2.5 and 5 T in a Carver hydraulic press using a punch of 9 mm diameter. The obtained tablets were broken down to powder and sent to PureCell Technologies inc. where the complex activity was tested.
PCT Stability to Compression in Presence of Polymeric Excipients
[0045]Tablets of 200 mg based on, one of the three polymers (alginate, CMCJ or CMC2) containing 20, 40 o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com