Use and production of storage-stable neutral metalloprotease
a neutral metalloprotease and production technology, applied in the field of use and production of storage-stable neutral metalloprotease, can solve the problems of difficult formulation and use of cleaning compositions, difficult to completely remove stains, and difficult to achieve the effect of improving storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
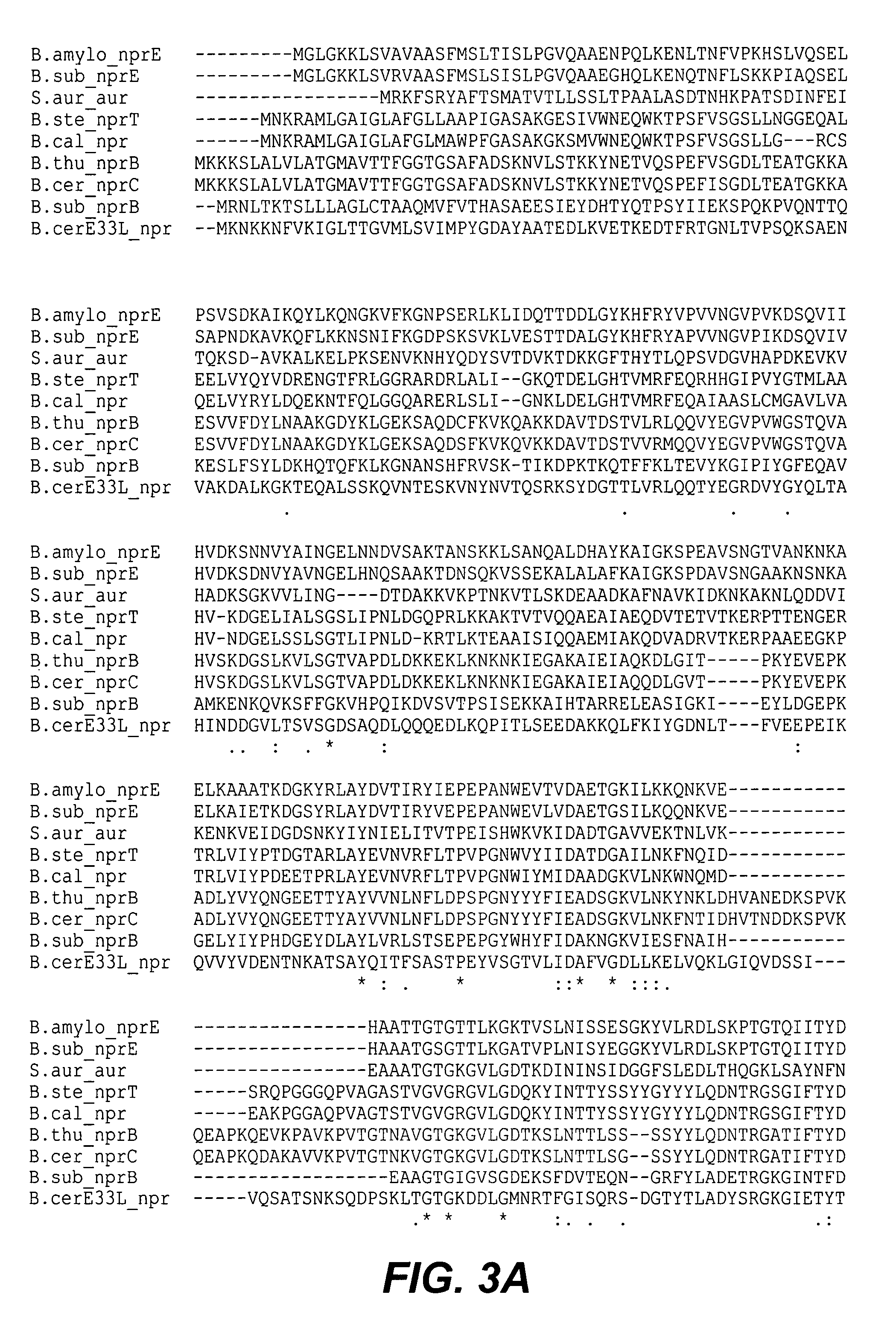
Cloning of the Neutral Metalloprotease Gene from B. amyloliquefaciens
[0277]In this Example, methods used to clone the B. amyloliquefaciens neutral metalloprotease gene are described. The gene-encoding neutral metalloprotease was cloned from B. amyloliquefaciens using well-established methods in this art. The non-exempt (i.e., the strain carries extrageneric DNA (besides the chloramphenicol selectable marker which is allowed in an exempt strain), specifically the plasmid pJM102 sequences) strain BC91504 (aprE / nprE-pJM102 in BG3594::comK) carries the B. subtilis aprE promoter and signal sequence fused to B. amyloliquefaciens nprE propeptide / mature gene in integrating plasmid pJM102.
[0278]The following two sequences (SEQ ID NO:1 and SEQ ID NO:2) of B. subtilis and B. amyloliquefaciens were generated via PCR with the oligonucleotide primers corresponding to the underlined sequences.
[0279]B subtilis chromosomal EcoRI restriction site (GAATTC) and aprE start codon (GTG) and B. amylolique...
example 2
Expression and Fermentation of the Purified MULTIFECT® Neutral and Recombinant Neutral Metalloprotease (nprE)
[0294]The recombinant Bacillus subtilis produced as described in Example I was cultivated by conventional batch fermentation in a nutrient medium as described below. One glycerol vial (prepared as described in Example 1) of B. subtilis culture containing the B. amyloliquefaciens neutral metalloprotease was used to inoculate 600 ml of SBG1% medium containing 200 mg / L chloramphenicol. The cultures were grown for 48 hours at 37° C., after which time, the culture fluid was recovered by centrifugation at 12,000 rpm, as known in the art. This procedure was done in duplicate. The final enzyme concentrations obtained were in the range of about 1.4 and 2 g / L.
example 3
Purification and Characterization of Neutral Metalloprotease
[0295]This Example describes the methods used to purify the neutral metalloprotease expressed by the organisms described in Example 2. After 36 hours of incubation at 37° C., the fermentation broth was recovered and centrifuged at 12 000 rpm (SORVALL® centrifuge model RC5B). The secreted neutral metalloproteases were isolated from the culture fluid and concentrated approximately 10-fold using an Amicon filter system 8400 with a BES (polyethersulfone) 10 kDa cutoff.
[0296]The concentrated supernatant was dialyzed overnight at 4° C. against 25 mM MES buffer, pH 5.4, containing 10 mM NaCl. The dialysate was then loaded onto a cation-exchange column Porous HS20 (total volume ˜83 mL; binding capacity ˜4.5 g protein / mL column; Waters) as described below. The column was pre-equilibrated with 25 mM MES buffer, pH 5.4, containing 10 mM NaCl. Then, approximately 200-300 mL of sample was loaded onto the column. The bound protein was el...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com