Manufacturing Method of High Purity Nickel, High Purity Nickel, Sputtering Target formed from said High Purity Nickel, and Thin Film formed with said Sputtering Target
a technology of high purity nickel and sputtering target, which is applied in the direction of magnetism of inorganic materials, magnetic materials, magnetic bodies, etc., can solve the problems of deterioration of mos-lsi interfacial quality, trouble at the interface bonding area, and inability to manufacture gas components such as carbon and oxygen. , to achieve the effect of efficient manufacturing of high purity nickel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
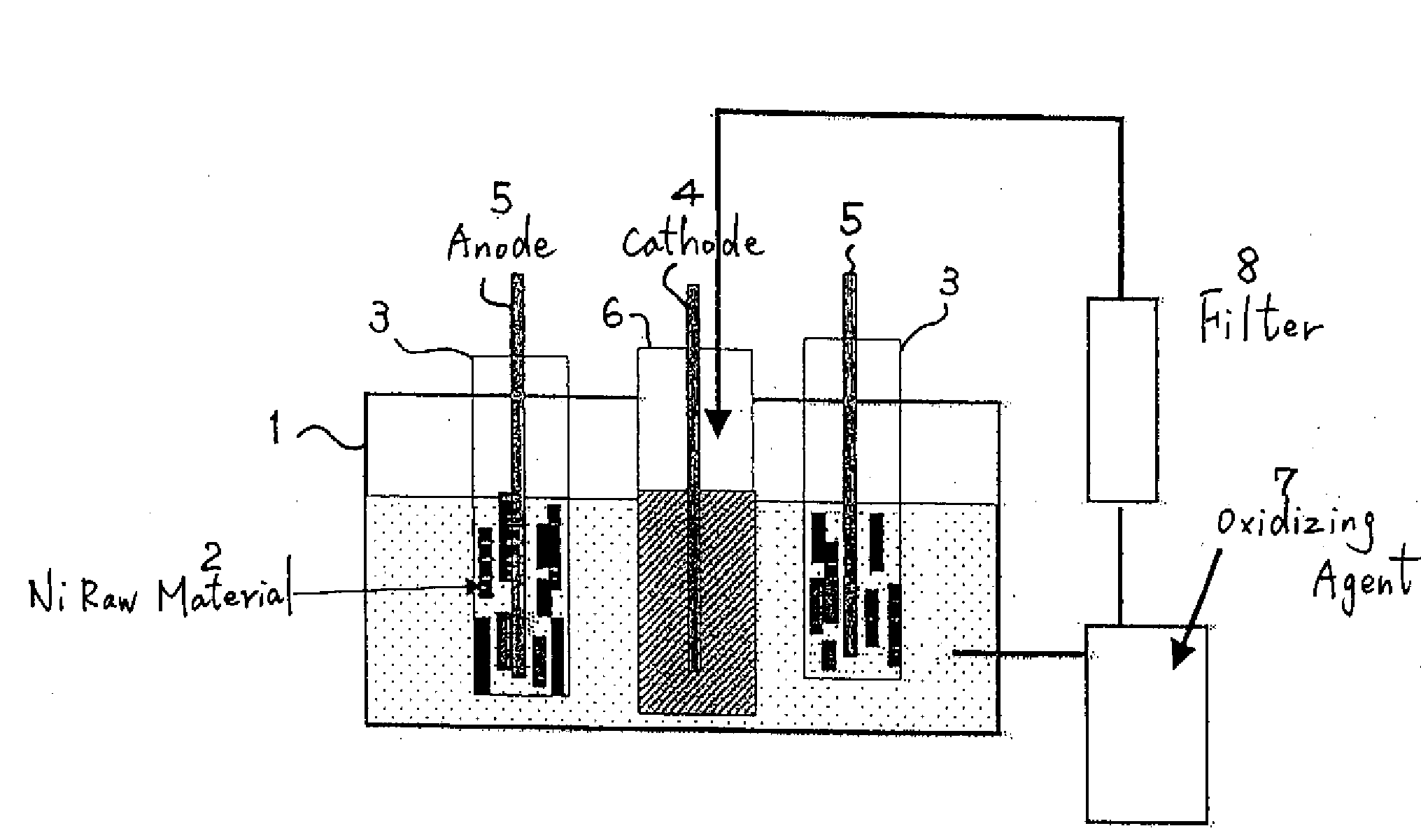
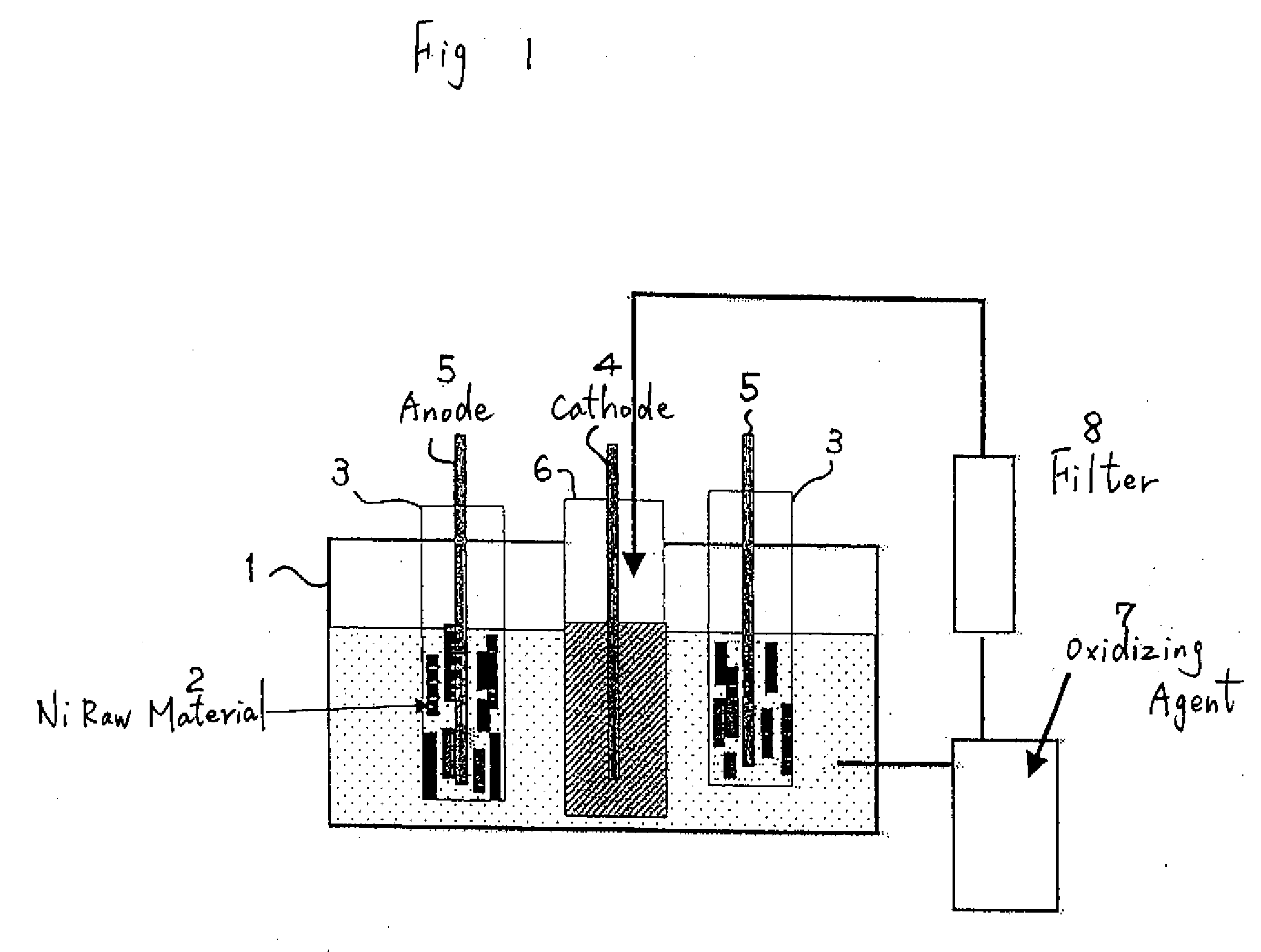
[0030]With the electrolytic bath shown in FIG. 1, 1 kg of massive nickel raw material of a 4N level was made an anode, and electrolysis was performed to a cathode with a nickel plate of a 2N level. The content of impurities in the raw material is shown in Table 1. The nickel raw material mainly contains a great amount of iron, carbon, oxygen, and so on.
[0031]Electrolysis was performed for 40 hr under a bath temperature of 50° C., in which 1 mol / L of hydrofluoric acid was added to a sulfuric acid electrolytic solution, a nickel concentration of 50 g / L and a current density of 2 A / dm2.
[0032]The solution pH was adjusted to 2. Here, anolyte was intermittently extracted. Hydrogen peroxide (H2O2) was added to the extracted anolyte to change the binary iron to ternary iron, and impurities such as iron were precipitated as hydroxide Fe(OH)3.
[0033]Further, impurities such as these sediments were eliminated with an activated carbon filter. According to the above, it was possible to make the c...
example 2
[0036]With the same electrolytic bath as Example 1, massive nickel raw material of a 4N level was made an anode, and electrolysis was performed to a cathode with a nickel plate of a 3N level.
[0037]Electrolysis was performed for 40 hr under a bath temperature of 30° C., with a hydrochloric acid electrolytic solution, a nickel concentration of 80 g / L and a current density of 5 A / dm2.
[0038]As with Example 1, the solution pH was adjusted to 2. Here, anolyte was intermittently extracted. Hydrogen peroxide (H2O2) was added to the extracted anolyte to change the binary iron to ternary iron, and impurities such as iron were precipitated as hydroxide Fe(OH)3.
[0039]Further, impurities such as these sediments were eliminated with an activated carbon filter. According to the above, it was possible to make the concentration of iron within the electrolytic bath to be 1 mg / L or less.
[0040]After the elimination of impurities, this solution was intermittently introduced to the cathode side; that is,...
example 3
[0050]1 kg of massive nickel raw material of a 3N level was made an anode, and electrolysis was performed to a cathode with an aluminum plate of a 2N level. The content of impurities in the raw material is shown in Table 2. The nickel raw material mainly contains a great amount of iron, carbon, copper, carbon, oxygen, and so on.
[0051]Electrolysis was performed for 25 hr under electrolytic conditions of a bath temperature of 40° C., in which 1 mol / L of hydrochloric acid was added to a sulfuric acid electrolytic solution, a nickel concentration of 100 g / L and a current density of 3 A / dm2.
[0052]The solution pH was adjusted to 2.5. Here, anolyte was intermittently extracted. Electrolysis was performed to the extracted anolyte in a preliminary electrolytic bath under a current density of 0.1 A / dm2, and impurities such as iron, cobalt and copper were eliminated.
[0053]Further, organic matter within the electrolytic solution was eliminated with an activated carbon filter. According to the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com