Atherectomy devices, systems, and methods
a technology of atherectomy and lumen, applied in the field of occluded body lumen treatment, can solve the problems of affecting the treatment effect of the leg, and the artery of the leg, and achieves the effects of improving the forward cutting speed, improving the conventional device, and excellent column strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0131]Although the disclosure hereof is detailed and exact to enable those skilled in the art to practice the invention, the physical embodiments herein disclosed merely exemplify the invention which may be embodied in other specific structures. While the preferred embodiment has been described, the details may be changed without departing from the invention, which is defined by the claims.
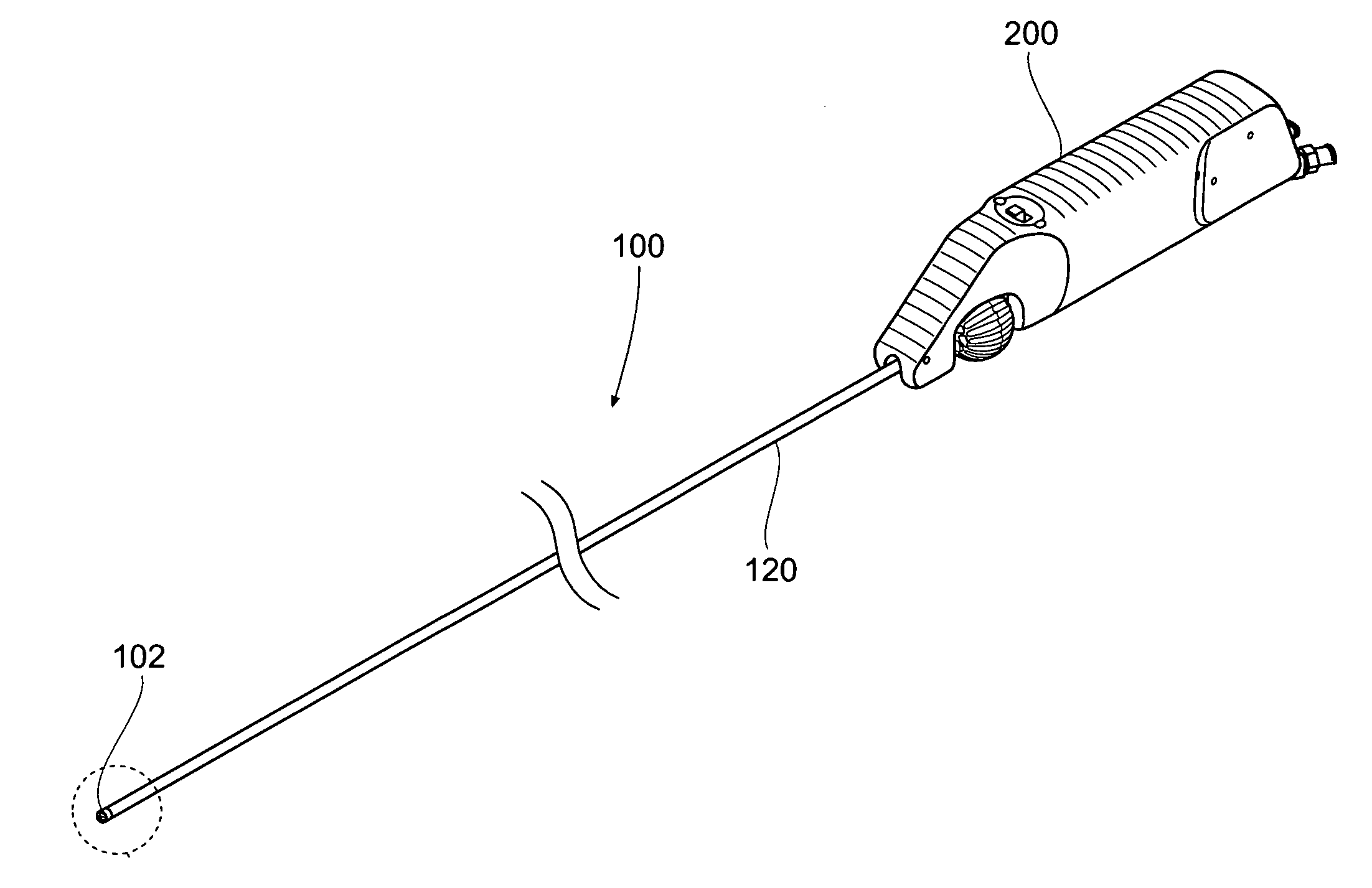
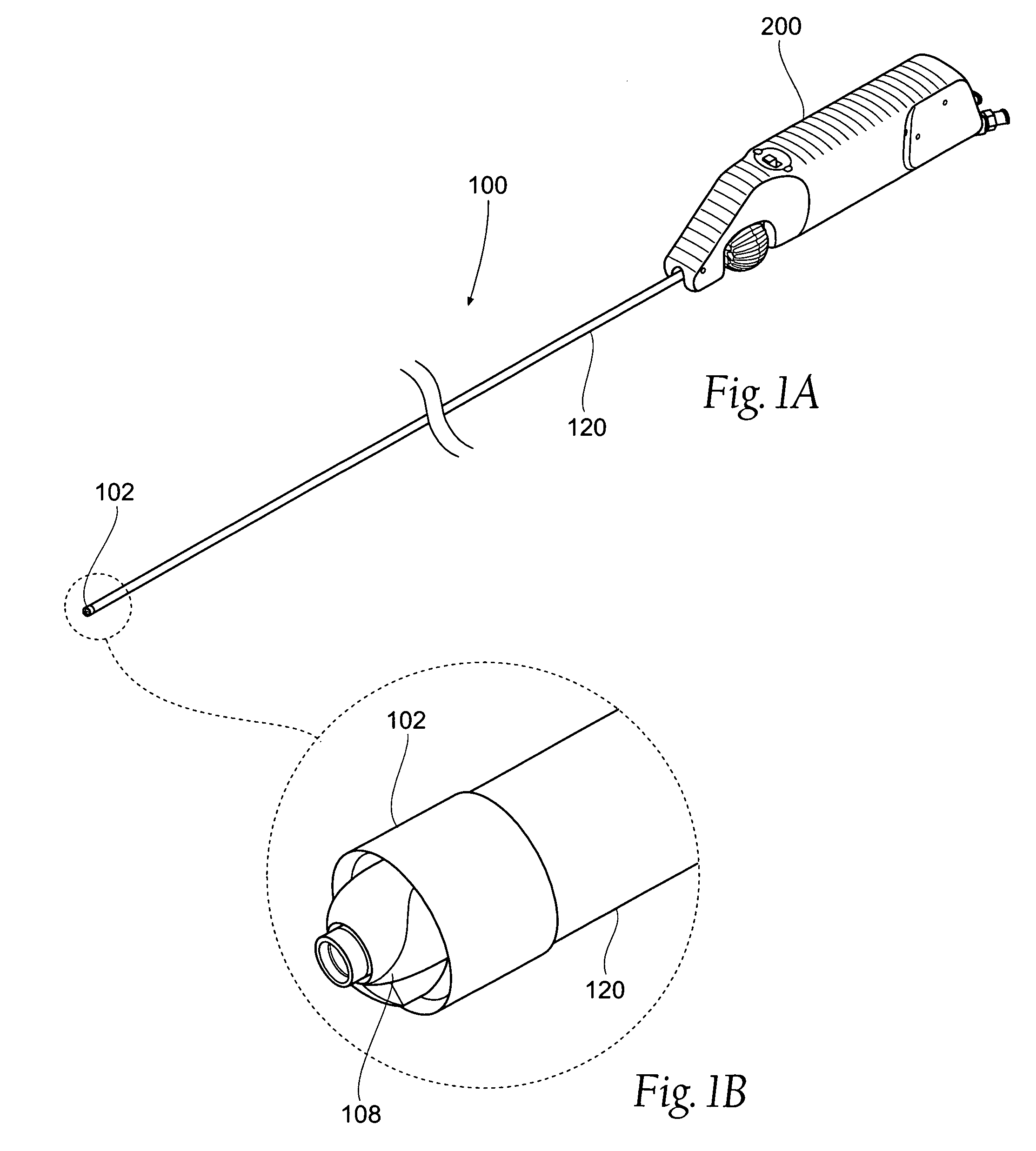
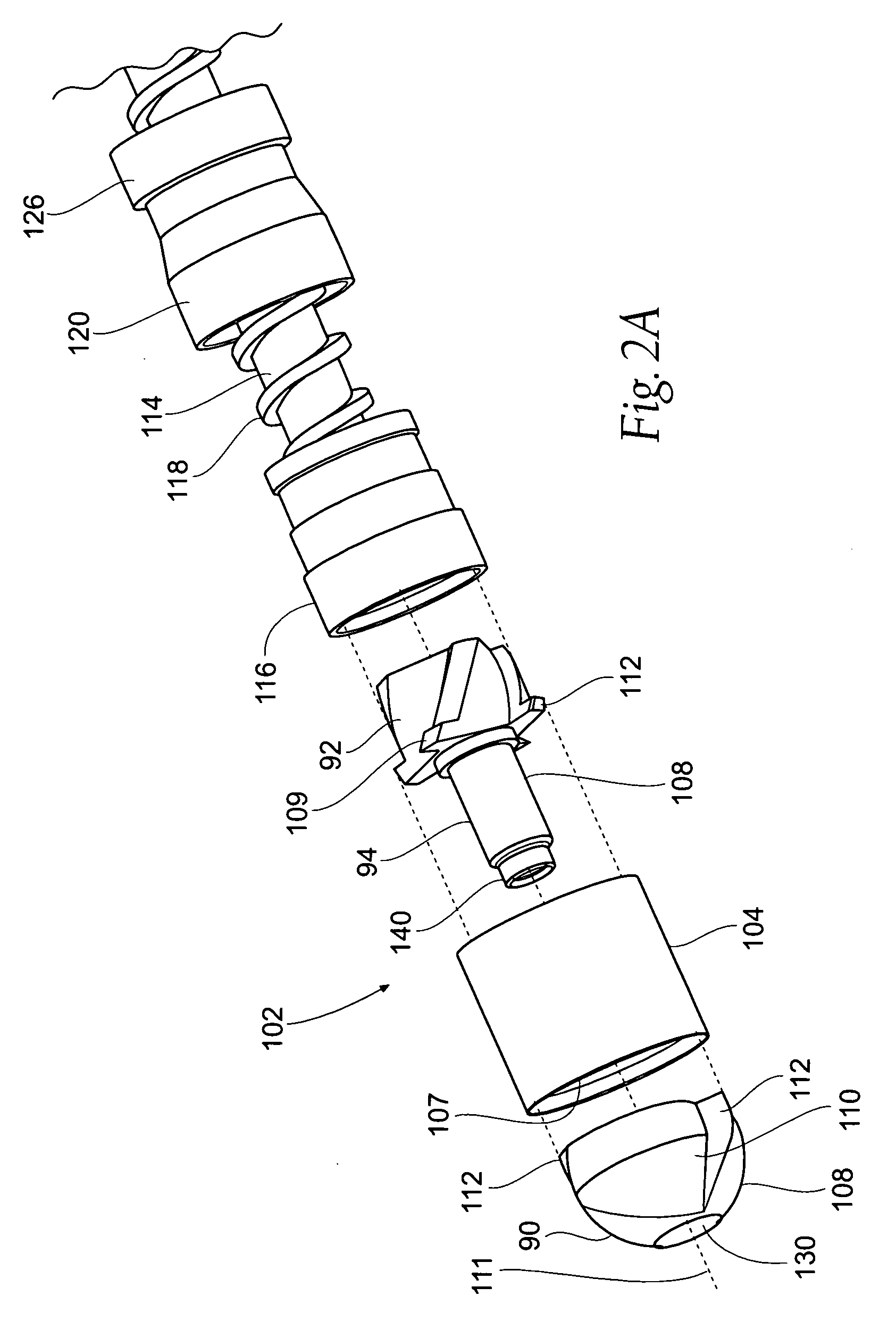
[0132]This specification discloses various catheter-based devices, systems, and methods for removing occluding materials from body lumens, including removing plaque, thrombus, calcium, and soft elastic tissues in blood vessels. For example, the various aspects of the invention have application in procedures requiring the treatment of diseased and / or damaged sections of a blood vessel. The devices, systems, and methods that embody features of the invention are also adaptable for use with systems and surgical techniques that are not necessarily catheter-based.
[0133]The devices, systems, and methods ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com