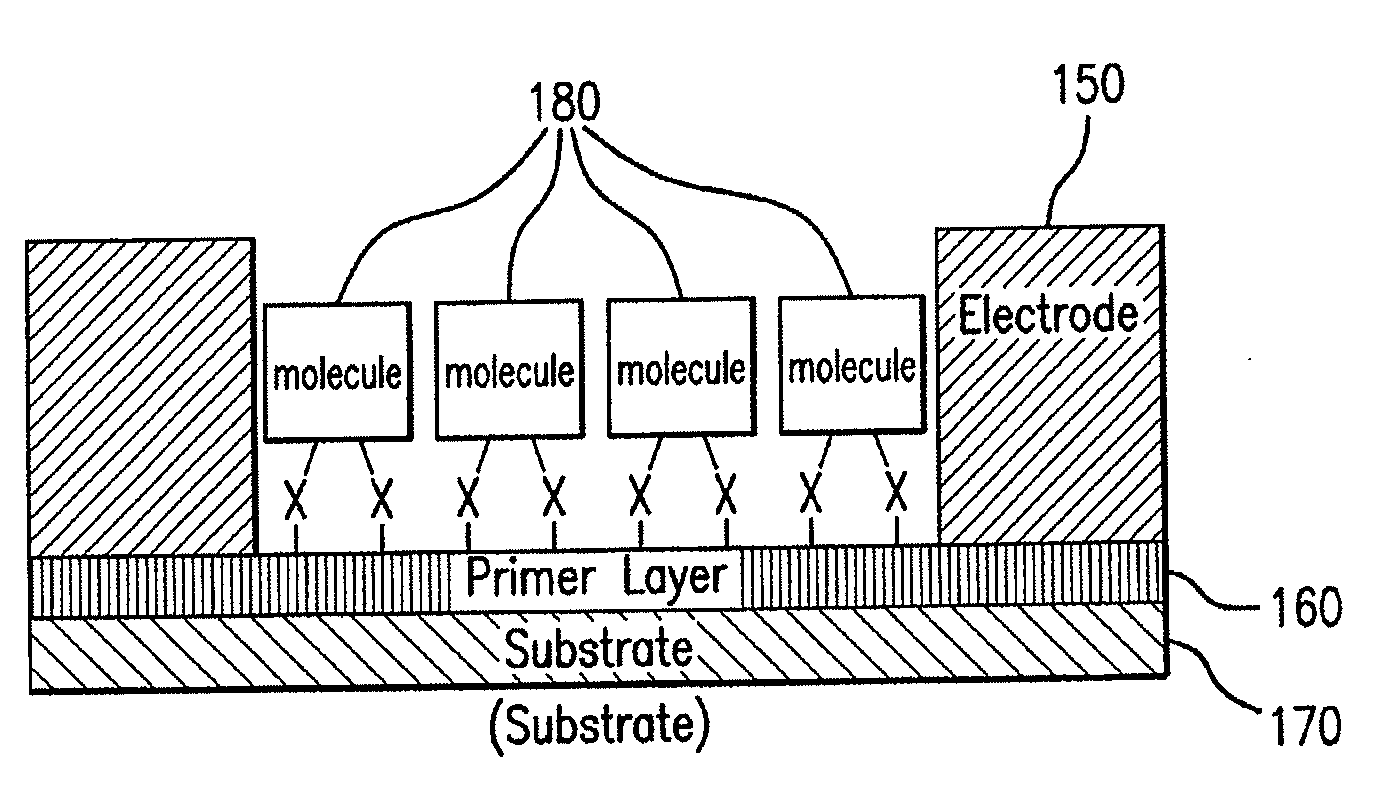
Sensing devices from molecular electronic devices utilizing hexabenzocoronenes
a technology of electronic devices and sensing devices, applied in nanoinformatics, instruments, coatings, etc., can solve the problems of difficult bonding between organic molecules and metal electrodes, difficult bridging, and great challenges in selecting appropriate molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Contorted Hexabenzocoronenes
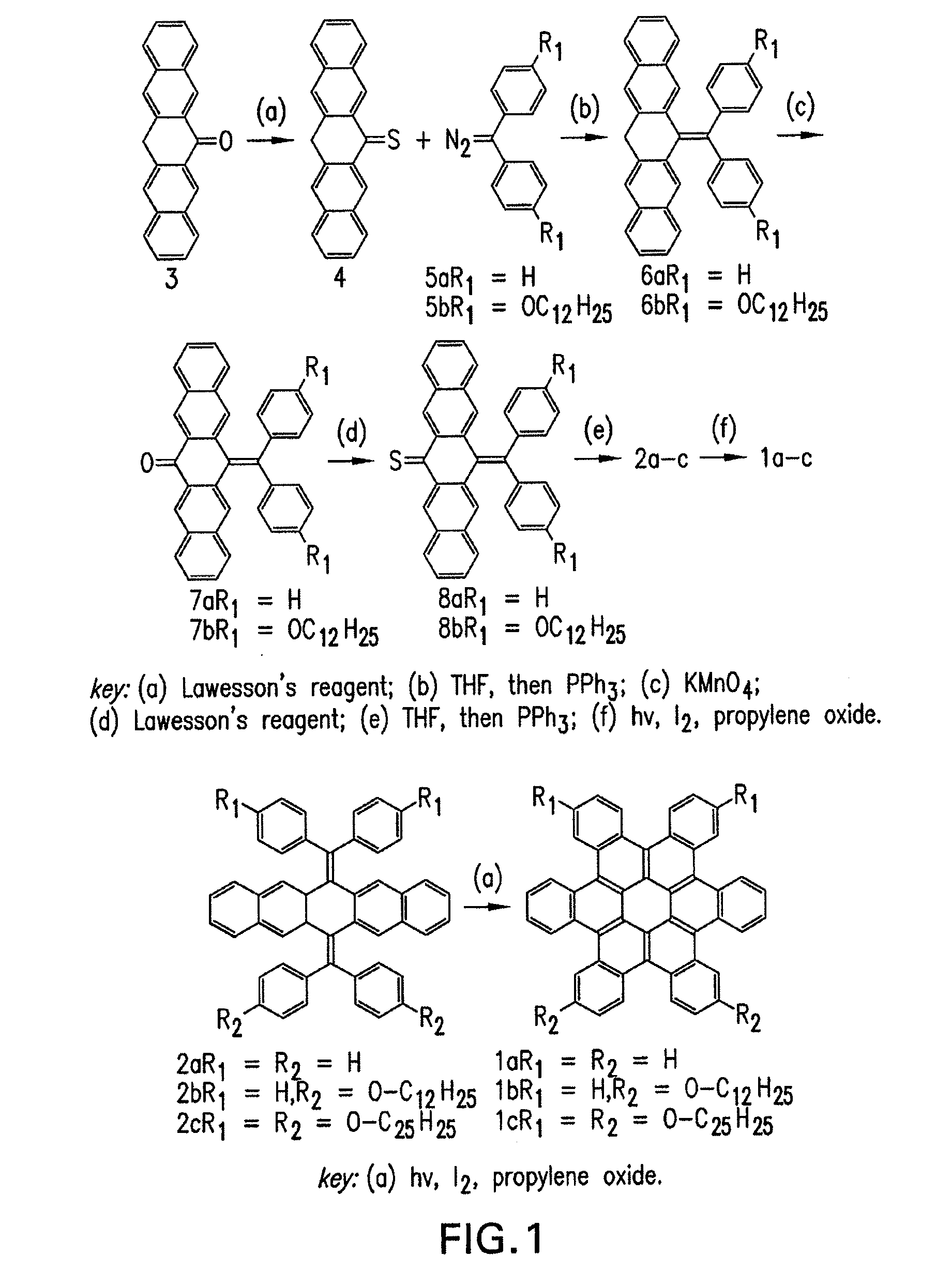
[0084]The syntheses for preparing contorted hexabenzocoronenes of formula 1 are schematically shown in FIG. 1. Ketone 3 was synthesized according to the well-known procedure disclosed in E. Clar,Chemische Berichte vol. 82, p. 495 (1949), the contents of which are incorporated by reference herein.
[0085]Synthesis of thioketone 4. Ketone 3 (4.4 g, 14.9 mmol) and Lawesson's reagent (0.7 eq, 4.2 g, 10.4 mmol) were added to 500 mL of toluene. The solution was heated to 80° C. for 2 hours. The dark green solution was allowed to cool to room temperature and 1200 mL of a 4:1 v / v mixture of hexanes and CH2Cl2 was added. Filtration through a plug of silica gel and a small amount of the same mixture of hexanes and CH2Cl2 was used to wash the remaining product from the silica gel. 3 was isolated as a green solid (2.2 g, 47%) after removal of the solvent and triturating with cold hexanes.
[0086]Synthesis of diphenyldiazomethane 5a: A mixture of 4,4′-dihyd...
example 2
Preparation of a Transistor Device Based on Contorted HBCs
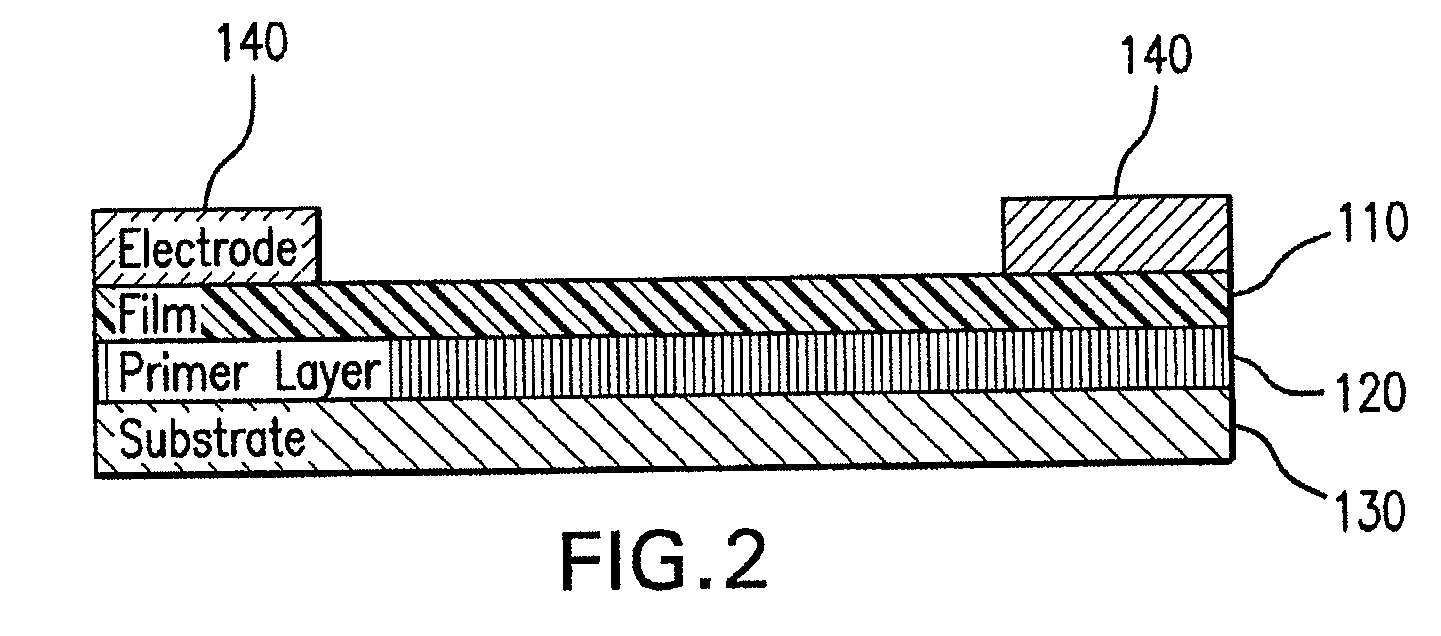
[0100]Compounds of the formula 1c were spin-cast from 1,2-dichloroethane or CHCl3 to form uniform films (approximately 100-nm thick) on top of a SiO2 substrate, and then Au was deposited as source and drain electrodes by thermal evaporation onto the spin-cast films through a metal-shadow mask. A transistor device thus obtained is illustrated schematically in FIG. 2. The transconductance and transistor output are shown in FIGS. 10A and 10B.
[0101]The mobility (0.02 cm2 V−1 s−1) shown in FIG. 10B is calculated from the linear portion of the data in FIG. 10B, and was based on a capacitance of 11.3 nF cm−2 for the gate dielectric layer of 300 nm of SiO2 and a monolayer of octadecyltrichlorosilane, obtained from a series of measurements over a range of frequencies. Other critical parameters, such as the threshold voltage for the device to turn on (as low as −3 V) and the on / off current ratios in the device (106:1), are also very go...
example 3
Fabrication of a Cut SWNT-Film Transistor Device
[0106]A compound of the formula 1c was spin cast onto the gap between a cut SWNT such that it covered gap of the cut SWNT but did not span the metal electrodes (shown in FIG. 15A). The device obtained shows p-type hole transporting semiconductor behavior (see FIG. 15B), but requires greater gate bias than a monolayer device where R2 is COCl is used where surface attachment is effected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| RF power | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com