Growth hormone secretion regulator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0072]
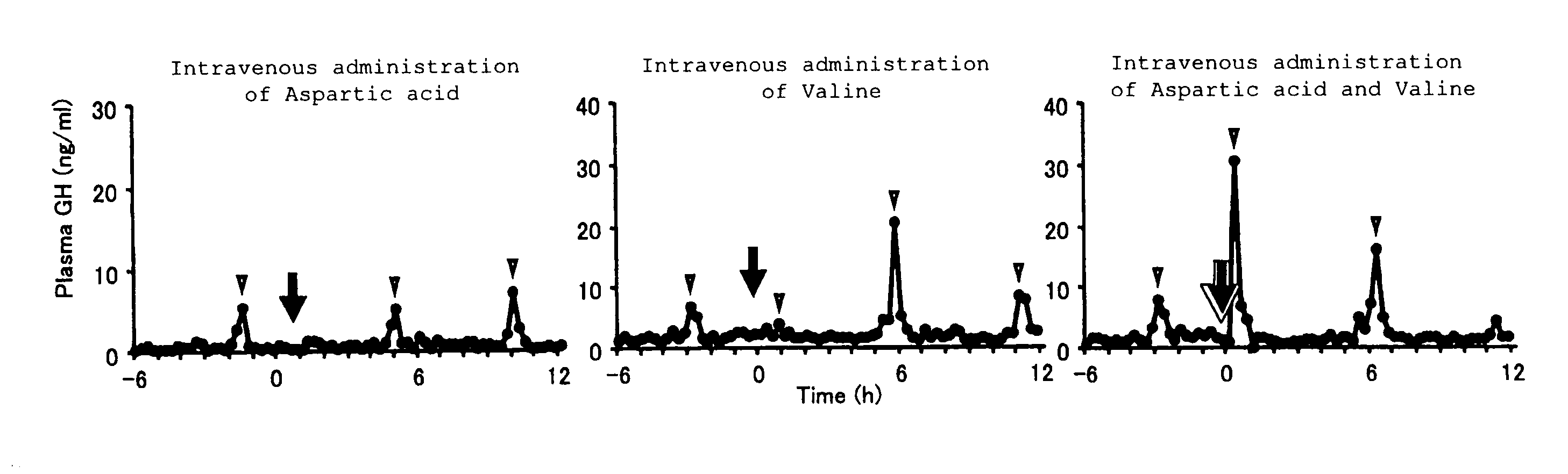
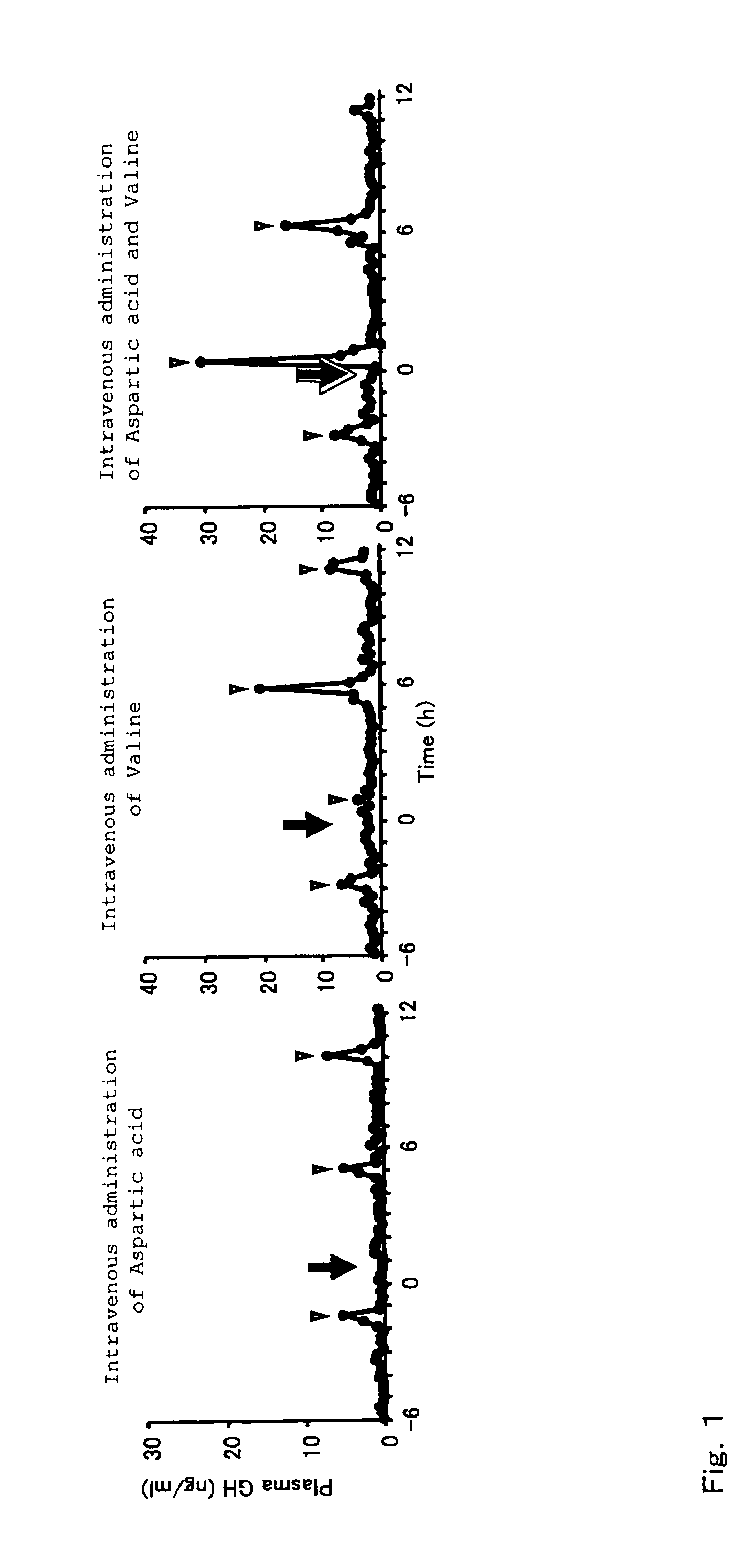
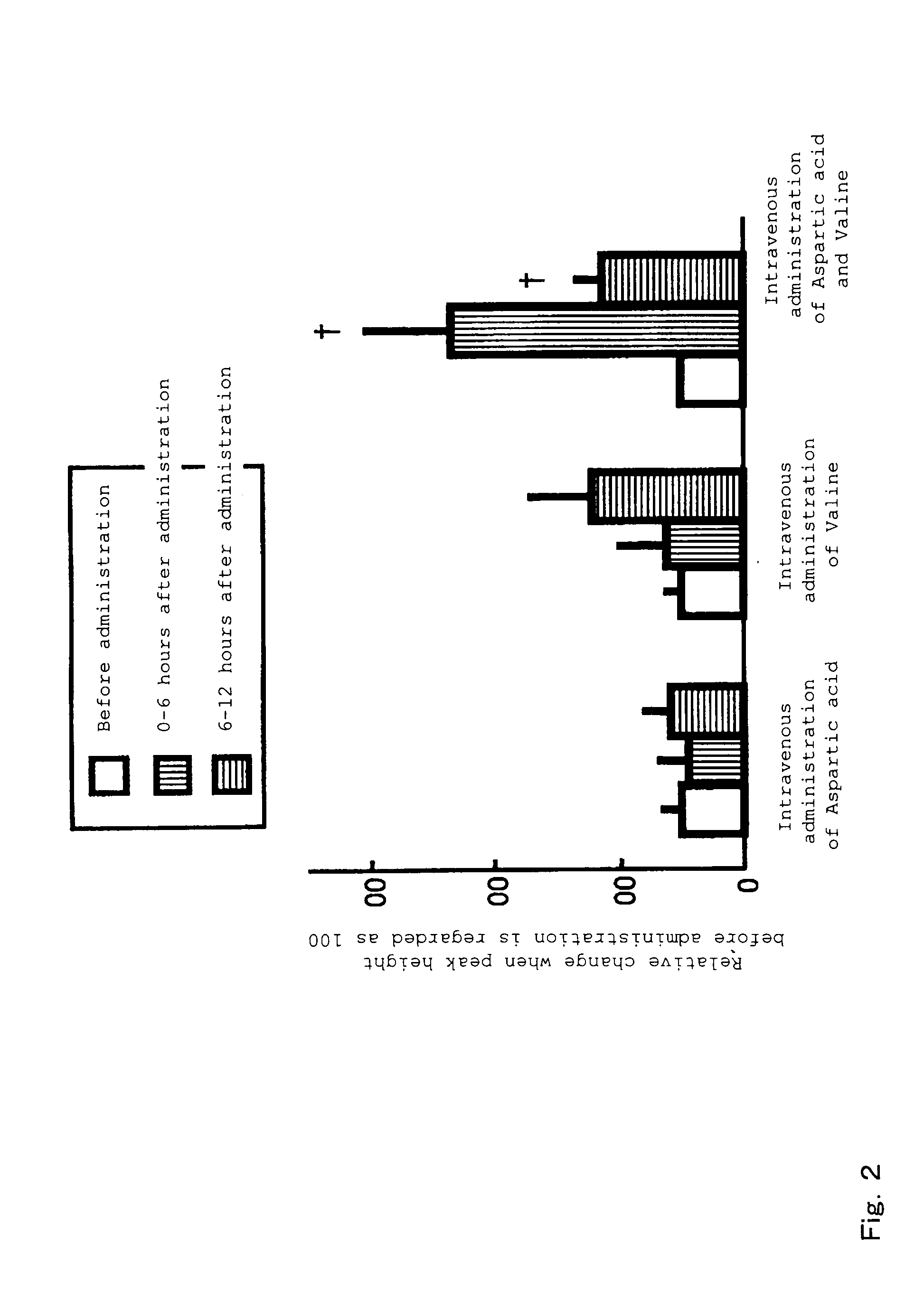
[0073]The growth hormone secretion regulating function of amino acids to be used in the present invention was evaluated by investigating changes in growth hormone secretion caused by intravenous administration of amino acid solutions. The details are as follows.
[0074]Special grade aspartic acid (Ajinomoto Co., Inc.) and valine (Ajinomoto Co., Inc.) were used as L-type amino acid samples. The amino acids were dissolved in purified water or an aqueous solution of 5% carboxymethylcellulose and administered as aqueous solutions (pH 7.0 to 8.0).
[0075]Two- to four-year-old mature castrated male and female shiba-goats (25 to 35 kg), purchased from the farm of Tokyo University, were domesticated for about one week. Aspartic acid (0.75 mmol / kg), valine (1.0 mmol / kg), and a mixture thereof, prepared in the same way as above, were administered intravenously via a cannula placed in the jugular vein, and blood was collected continuously during a period between 6 hours before and 12 hours a...
example 2
[0078]
[0079]Two- to four-year-old mature castrated male and female shiba-goats (25 to 35 kg), purchased from the farm of Tokyo University, were domesticated for about one week. Sodium aspartate monohydrate (5 mmol / kg, 861 mg / kg), valine (5 mmol / kg, 586 mg / kg), and a mixture thereof, prepared in the same way as Example 1, were administered orally to the shiba-goats, and blood was collected continuously during a period between 6 hours before and 12 hours after administration every 15 minutes via a cannula placed in the jugular vein, followed by investigation of changes in growth hormone secretion (n=3). The concentrations of the growth hormone in blood were measured by immunoassay in which europium was used.
[0080]The growth hormone and antibody used in the immunoassay were purchased from National institute of Diabetes and Digestive and Kidney Diseases. The other procedures were performed based on a reference (Sugino T et al., Biochemical and biophysical research communications 295: 25...
example 3
[0082]
[0083]Diabetes and hyperglycaemia model animals, db / db mice (8-week-old, 10 mice in total, average body weight 43.9 g) were purchased from Charles River Laboratories, Inc. and were domesticated for about one week before an experiment. A mixture obtained by blending sodium aspartate monohydrate and valine in a 5% carboxymethylcellulose solution using an agate mortar (sodium aspartate monohydrate 751 mmol / l, and valine 427 mmol / l) was administered orally using a feeding needle to five of the mice once a day on weekdays 10 a.m. to 11 a.m. (10 ml / kg, that is, sodium aspartate monohydrate 1.0 g / kg and valine 0.5 g / kg).
[0084]Only a solvent was administered orally to five of the control-administered group in the same manner as above. Administration was continued for two weeks, daily body weight measurement and blood collection immediately once a week before administration were performed to measure blood sugar levels in full feeding and concentrations of insulin-like growth factor I i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Surface roughness | aaaaa | aaaaa |
| Mass ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com