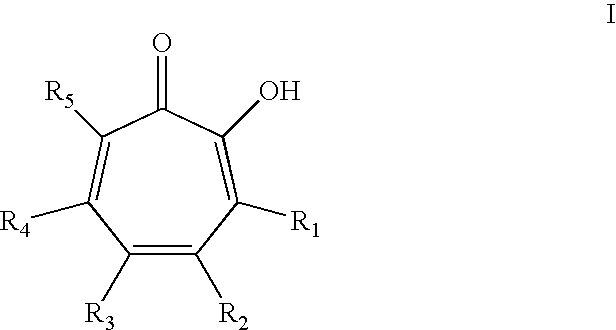
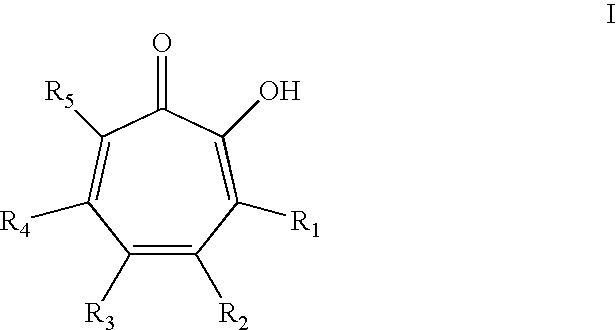
Oral Care Compositions Comprising Tropolone Compounds and Essential Oils and Methods of Using The Same
a technology of tropolone compounds and oral care compositions, which is applied in the field of oral care compositions to achieve the effects of suppressing or eliminating, high antimicrobial kinetics and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mouthwash Composition Containing Compounds of Formula (I) and Essential Oils
[0079]A mouthwash composition was prepared having the following components shown below in Table 1.
TABLE 1Components% by weight1)Thymol0.0642)Eucalyptol0.0923)Methyl Salicylate0.0604)Menthol0.0425)Alcohol, USP21.66)Compound of Formula (I)0.037)Flavoring Oil0.0858)Poloxamer 4070.159)Benzoic Acid0.1510)Sorbitol2011)Saccharin0.11712)FD&C Green #30.000513)n-Propanol0.514)Water, USPQS to 100
[0080]The composition was prepared by adding the essential oils (thymol, menthol, methyl salicylate and eucalyptol) and the compound of Formula (I), flavoring oils, poloxamer 407 and benzoic acid to alcohol followed by addition of 250 ml of water. Sorbitol, saccharin, and the dye were added to the resulting mixture followed by the addition of water to provide a 1000 ml sample of the mouthwash composition.
example 2
[0081]
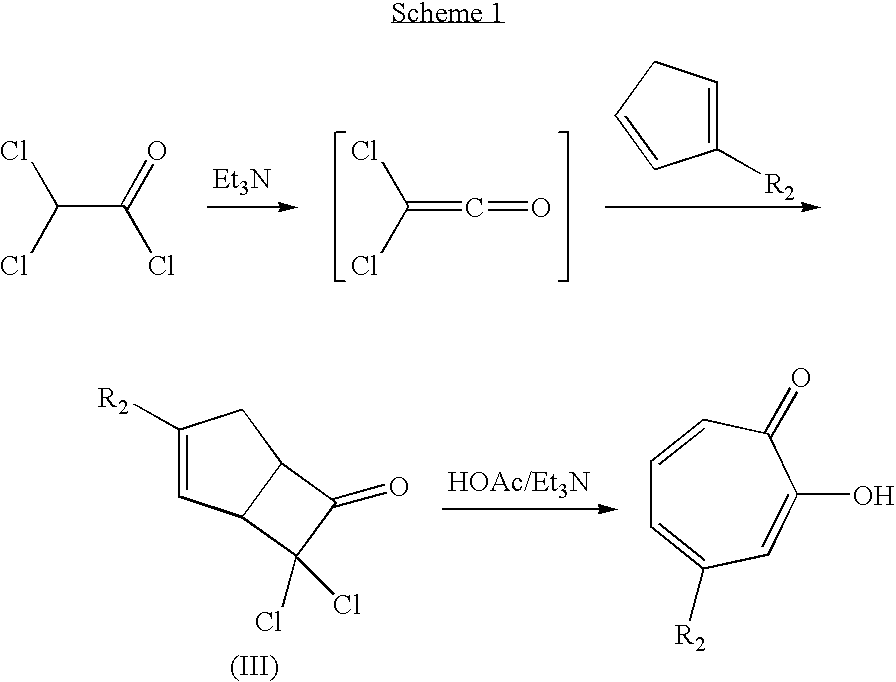
[0082]As illustrated in Scheme A, a solution comprising 25 mL of triethylamine and 100 mL of a mixture of hexanes was added dropwise to a solution containing 48.5 g of methylcyclopentadiene (prepared from cracking methylcyclopentadiene dimer) and 22.5 g of dichloroacetyl chloride in 200 mL of a mixture of hexanes at 0° C. The mixture was stirred for about one hour and thereafter poured into 150 mL of water at about 0° C. The layers were separated and the aqueous phase was extracted with two 75 mL portions of hexanes. The combined organic layers were washed twice with water, then dried over Na2SO4 and concentrated to yield an oil. Fractional distillation was performed under vacuum to yield a cycloadduct of Formula (III), which was dissolved in 80 mL of acetone. The resulting solution was then added to a solution containing 28.5 mL of acetic acid, 82.5 mL of triethylamine, 36 mL of water and 100 mL of acetone. The mixture was refluxed under nitrogen for about four hours. The ace...
example 3
Synthesis of 4-Isopropyl-7-methoxymethyltropolone
[0083]
[0084]As illustrated in Scheme B, a solution comprising 10 g of hinokitiol and 18.8 mL of 25% aqueous KOH was heated to 60° C. under argon. Formaldehyde was added in 1 mL portions every 30 minutes until a total of 10 mL was added. The reaction mixture was concentrated to a yellow paste and 300 mL of acetone was added. The resulting yellow solid was collected and washed with acetone. The solid was suspended in 250 mL in dichloromethane and 50 mL of 2.0M HCl was added. The aqueous layer was washed with dichloromethane and the combined organic layers were washed with water, and then dried over Na2SO4. The solvent was removed under vacuum leaving an oil which solidified upon trituration with petroleum ether. An ice cold solution of 4.0 g of 4-isopropyl-7-hydroxymethyltropolone and 1.8 mL of pyridine in 100 mL of diethyl ether was vigorously stirred for two hours as a solution of SOCl2 (2.5 mL) in 80 mL of ether was added thereto. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com