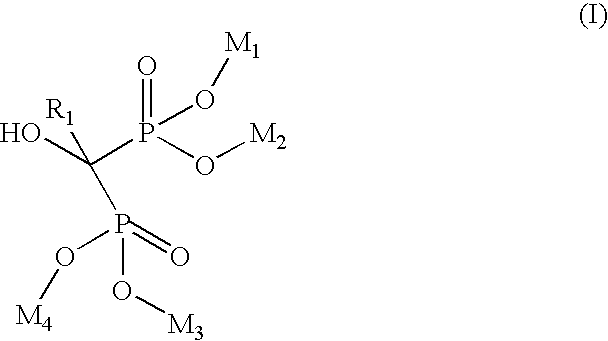
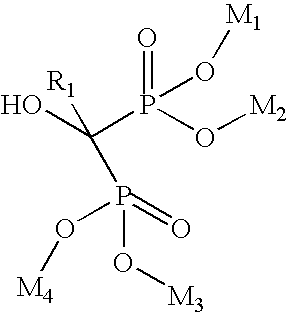
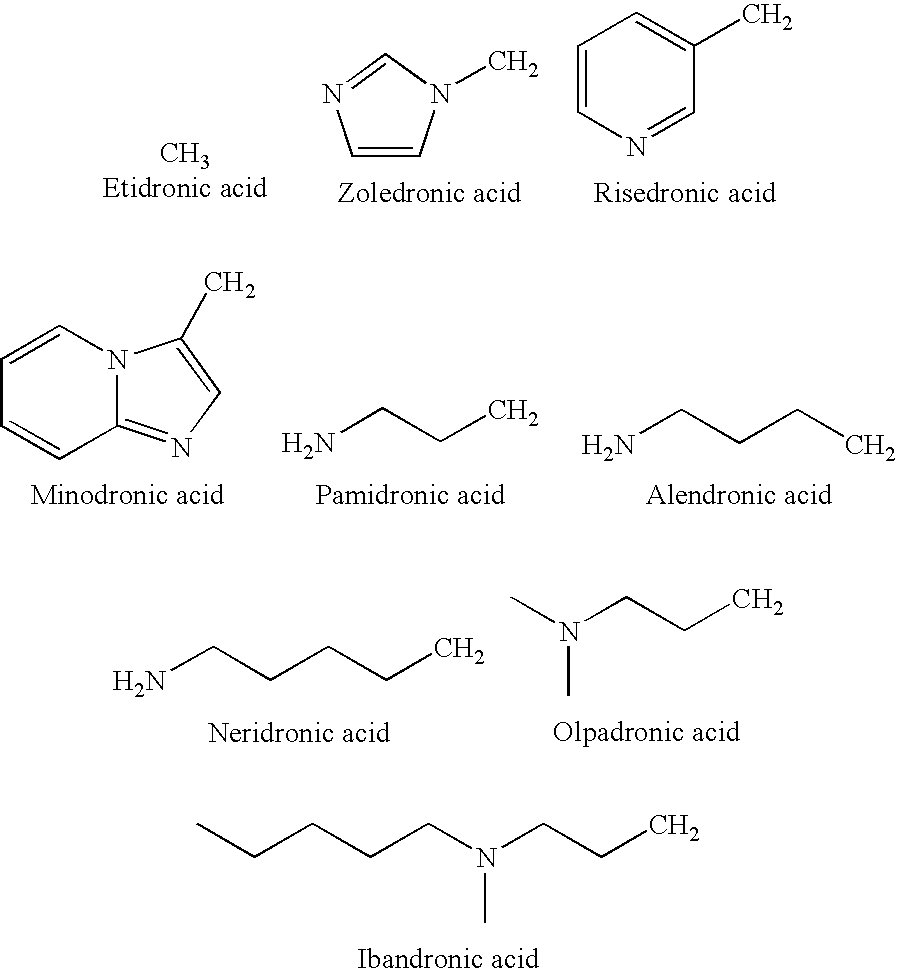
Process for manufacturing bisphosphonic acids
a technology of bisphosphonic acid and industrial process, which is applied in the field of improved industrial process for the preparation of bisphosphonic acid, can solve the problems of uncontrolled exotherm, safety concerns, and solidification of reaction melt, and achieve the effect of easy adaptation to manufacture other bisphosphonic acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Imidazoleacetic Acid
[0016]A 50 L reactor was charged with chloroform (54 kg), imidazole (6.13 kg, 90.04 mol) and t-butyl chloroacetate (5.48 kg, 36.4 mol). The temperature was increased to 60° C. over a 2 hour period and maintained at 60° C. for an additional 24 hours. The reaction mass was cooled to room temperature. The chloroform phase was washed successively with four portions of water (7.2 kg each) to remove imidazolium salts and excess imidazole.
[0017]Water (15.1 kg) was added and chloroform was removed by distillation with a jacket temperature of 60-65° C. (53° C. is the boiling point of the azeotrope) using a Dean-Stark trap to return the water phase. After chloroform was removed, the reactor jacket temperature was slowly raised to 115° C. during which time t-butanol and water co-distilled (azeotrope boiling point is 80° C.). After the alcohol was removed, the aqueous solution was cooled and drained from the reactor, giving 17.54 kg of solution containing imid...
example 2
Isolation of Imidazoleacetic Acid
[0018]A portion of the solution from Example 1 (1.13 kg) was rotary evaporated to give a slurry of solids (0.38 kg) to which was added acetone (234 g) to complete crystallization. The solid was filtered, washed with acetone and dried with a stream of nitrogen. The evaporator condensate was re-evaporated, washed and dried to give a second crop of crystals; this was combined with the first, to give imidazoleacetic acid (219 g, 91% recovery, 98.9 wt % pure by NMR assay). 1H NMR (D20): 8.68 (s, 1H); 7.42 (s, 2H); 4.83 (s, 2H); 4.79 (br s, 1H).
example 3
Preparation of Zoledronic Acid
[0019]A 1.5 liter kettle reactor, fitted with a heating mantle, mechanical stirrer, dropping funnel, thermocouple and condenser with nitrogen inlet adapter, was charged with imidazoleacetic acid (100 g, 0.793 mol), diglyme (400 ml), and 85% phosphoric acid (55 ml). Phosphorus trichloride (330 g, 2.41 mol) was slowly added to the reaction mass resulting in an exotherm and the evolution of hydrogen chloride. The temperature was allowed to rise to 70° C. and the solution was stirred until the evolution of HCl subsided. The temperature of the reaction mass was increased to 85° C. and a white solid began to form, float and adhere to the stirrer shaft. After about 1 hour, stirring became impossible and the stirring motor was stopped. The reaction mass was heated for 5 more hours at 85° C. and then cooled to ambient temperature, producing a solid homogeneous white mass.
[0020]Water was slowly added to the white mass (320 ml) that resulted in an exotherm and HCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com