Antibody fragment-targeted immunoliposomes for systemic gene delivery
a technology of immunoliposomes and antibody fragments, which is applied in the field of preparation of antibody fragment-targeted liposomes, to achieve the effects of easy incorporation of scfv into liposomes, high transfection efficiency, and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction and Expression of Biosynthetically Lipid-Tagged scFv
1. Construction of the Expression Vector for TfRscFv
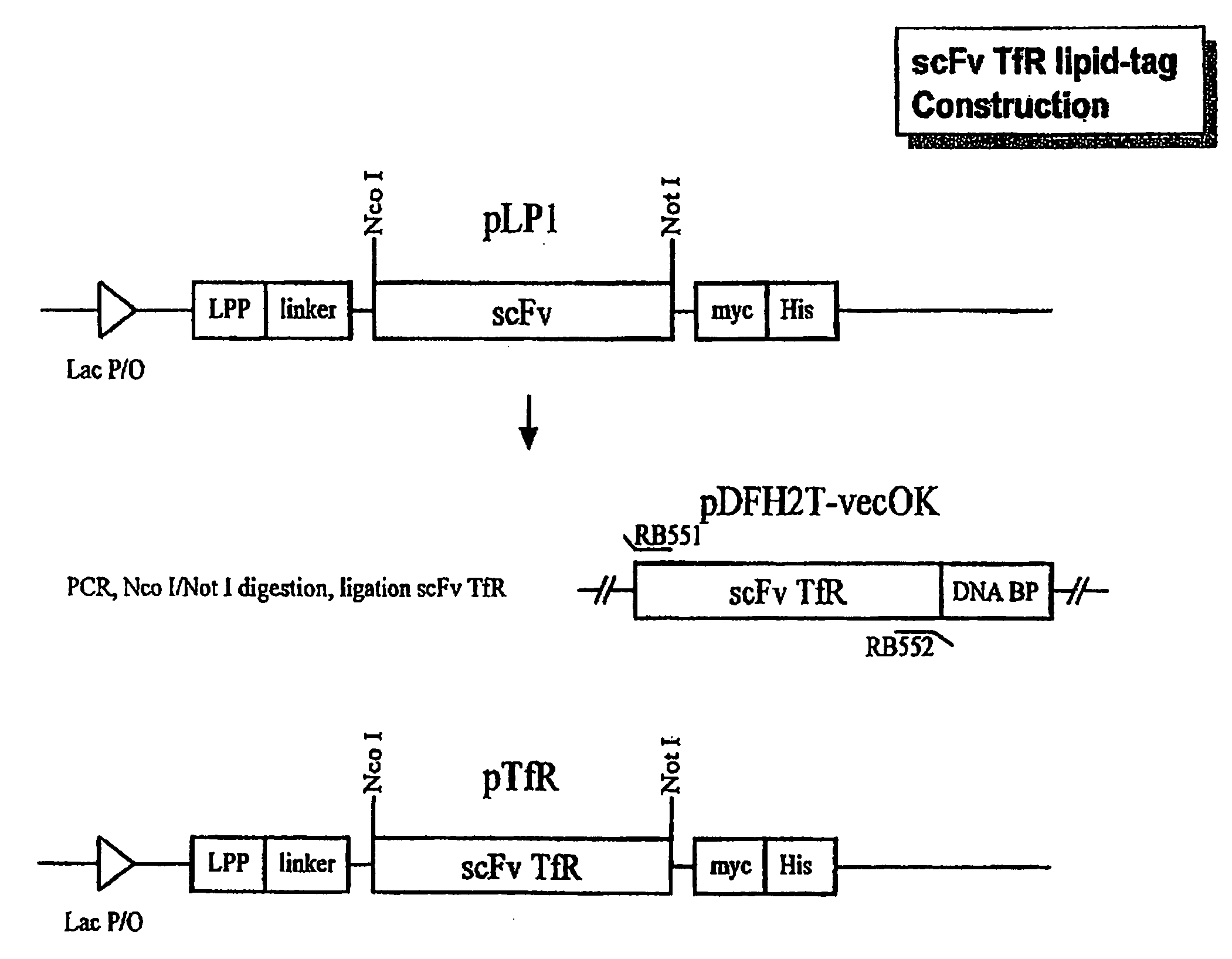
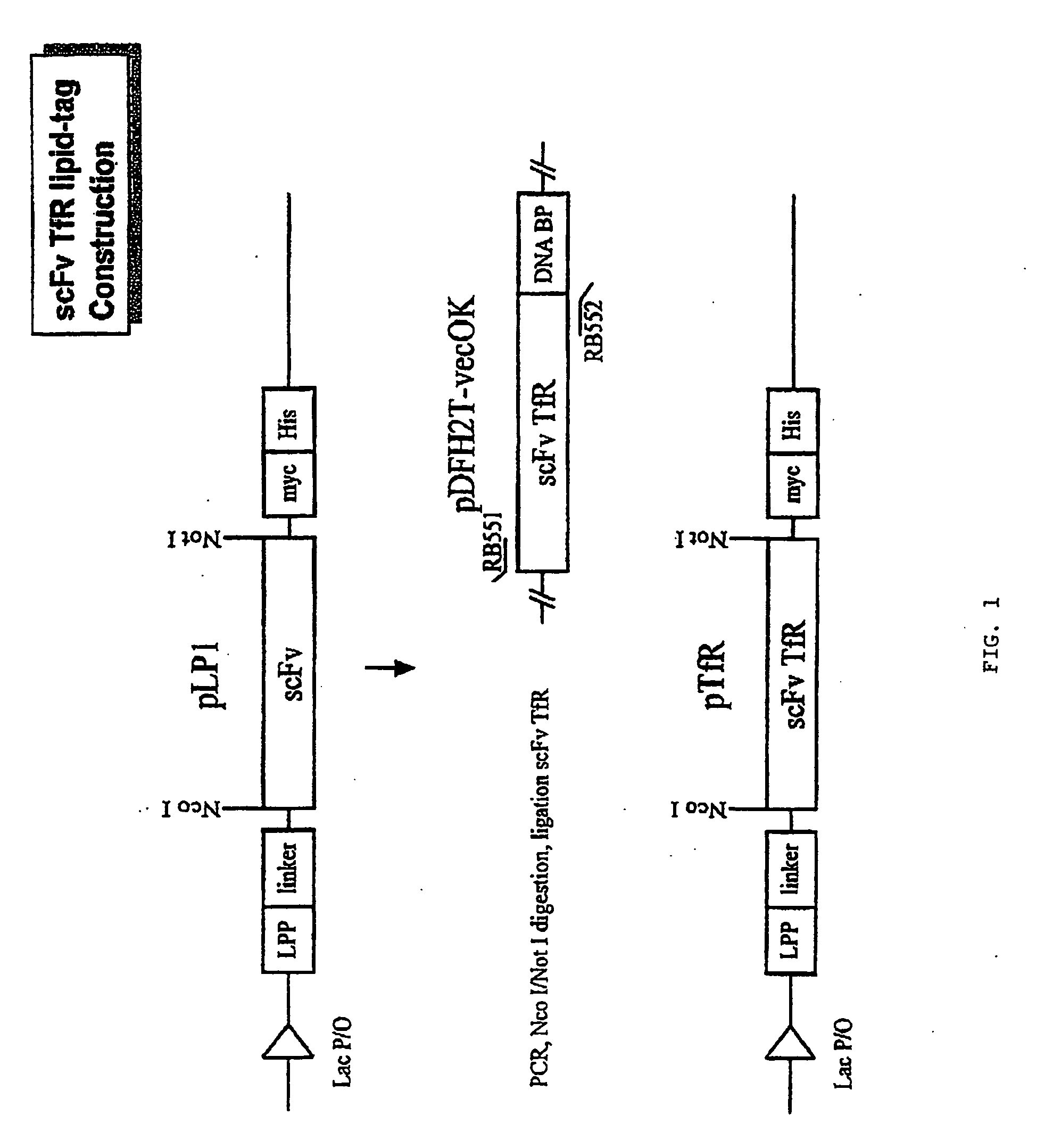
[0031]To construct the expression vector, we used the vector pLP1 which contains an amino acid linker sequence between the E. coli lipoprotein signal peptide (ssLPP) and the scFv cloning site (de Kruif et al., FEBS Lett. (1996) 399:232-236). This vector contains both c-myc and His6 tag sequences that can be used for purification and detection of the expressed scFv (FIG. 1).
[0032]We obtained a plasmid expression vector, pDFH2T-vecOK, which contains the single chain fragment for the 5E9 (Haynes et al., J. Immunol. (1981) 127:347-351) antibody linked to a DNA binding protein, which recognizes the human transferrin receptor (TfR). This vector also contains the sequence for a DNA binding protein, and there are no unique restriction enzyme sites flanking the scFv sequence in pDFH2T-vecOK. Therefore, we cloned the VH-linker-Vκ scFv by PCR amplification of the desired fragmen...
example 2
Preparation of Lipid-Tagged ScFv-Immunoliposomes by a Lipid-Film Solubilization Method
[0034]This example discloses a detailed procedure of lipid-film solubilization method to prepare lipid-tagged scFv-immunoliposomes. 5 μmol lipids (DOTAP / DOPE, 1:1 molar ratio) in chloroform are evaporated under reduced pressure to obtain a dry lipid film in a glass round-bottom flask. To the lipid film is added 0.5 ml 1% OG, 20 mM HEPES, 150 mM NaCl, pH 7.4, containing the lipid-modified scFv. This is incubated 10-20 minutes at room temperature and then vortexed to solubilize the lipid membrane. 2 ml sterile water is then added to dilute the scFv-lipid mixture. The solution is briefly sonicated to clarity in a bath-type sonicator at 20° C. The scFv-liposome is a clear solution with a limited amount of detergent OG left. The OG and the uncomplexed scFv can be eliminated by chromatography with Sepharose CL-4B or Sephacryl S500, even though they do not interfere a lot with the subsequent use.
example 3
Preparation of Lipid-Tagged ScFv-Immunoliposomes by a Direct Anchoring Method
[0035]This example provides a direct anchoring method to prepare lipid-tagged scFv-immunoliposomes. 20 μmol lipids (LipA-H, see below for compositions and ratios) prepared as dry lipid film in a glass round-bottom flask is added to 10 ml pure water and sonicated in a bath-type sonicator for 10-30 min at room temperature (LipA, B, C) or at 65° C. (LipD, E, G, H, or any composition with Cholesterol (Chol)). The cationic liposomes prepared are clear solutions, their compositions and ratios are as follows:
LipADOTAP / DOPE1:1 molar ratioLipBDDAB / DOPE1:1 molar ratioLipCDDAB / DOPE1:2 molar ratioLipDDOTAP / Chol1:1 molar ratioLipEDDAB / Chol1:1 molar ratioLipGDOTAP / DOPE / Chol2:1:1 molar ratioLipHDDAB / DOPE / Chol2:1:1 molar ratio
[0036]For attaching the scFv to preformed liposomes, the lipid-modified scFv (Ipp-scFv) in 20 mM HEPES, 150 mM NaCl, pH 7.4, containing 1% OG is added to preformed liposomes while vortexing, at volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com