Method for treating joint damage
a joint damage and joint technology, applied in the field of joint damage treatment, can solve the problems of joint damage and damag, poor long-term prognosis of ra, and loss of daily function, and achieve the effect of reducing joint damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Efficacy and Safety of Rituximab in Patients with an Inadequate Response or Lack of Tolerance to Prior Anti-TNF Therapy
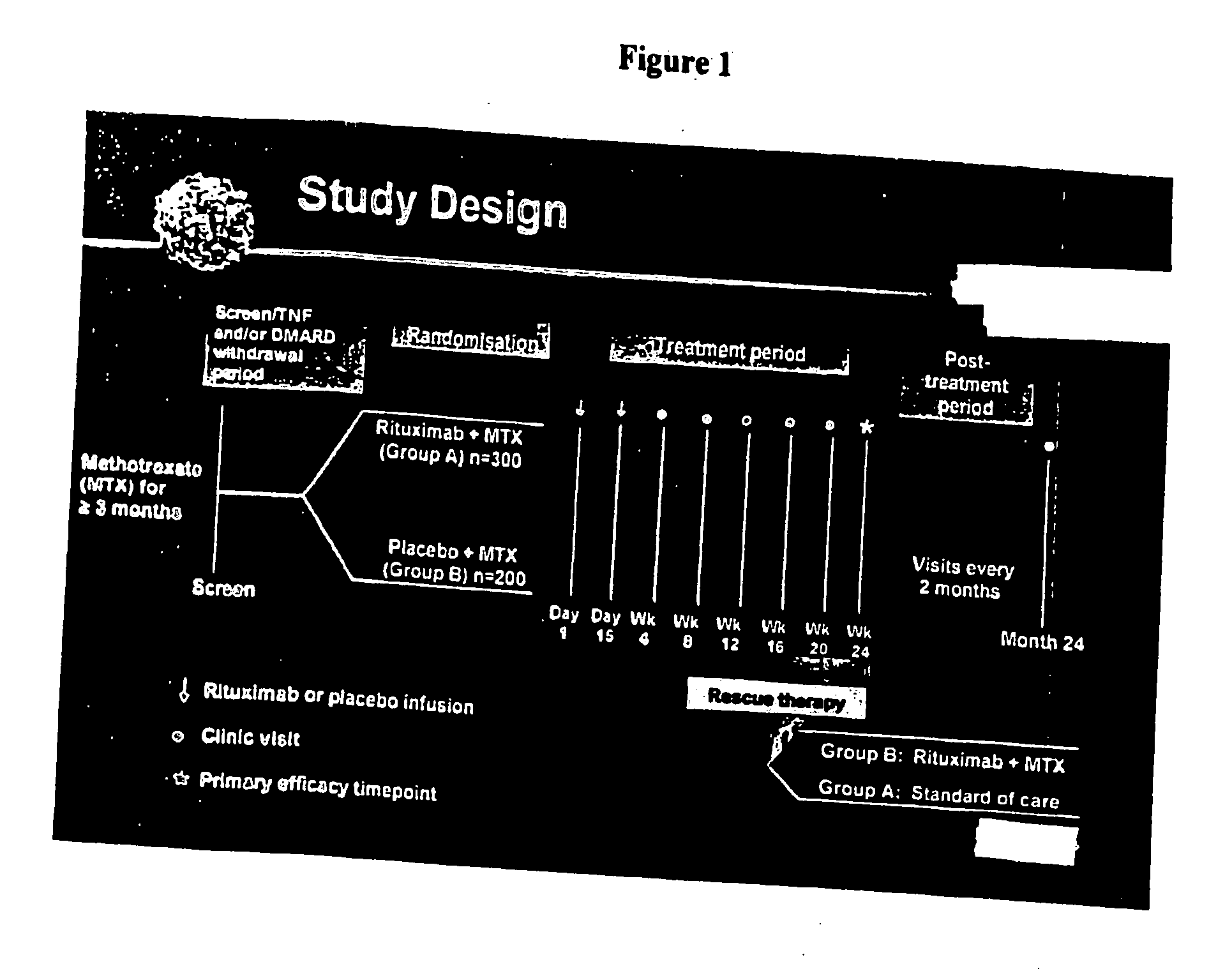
[0351]This is a Phase III randomized, double-blind, parallel-group multicenter clinical trial, called: Randomised Evaluation oFLong-term Efficacy of Rituximab in RA (REFLEX). The study design is shown in FIG. 1.
The objectives of this study were:[0352]To determine the efficacy and safety of rituximab when used in combination with methotrexate (MTX) in 517 patients with active rheumatoid arthritis who have an inadequate response to one or more anti-TNF therapies.[0353]To explore the pharmacokinetics and pharmacodynamics of rituximab in this patient population (e.g. extent of duration of B-cell depletion and effects on immunoglobulins and rheumatoid factor).
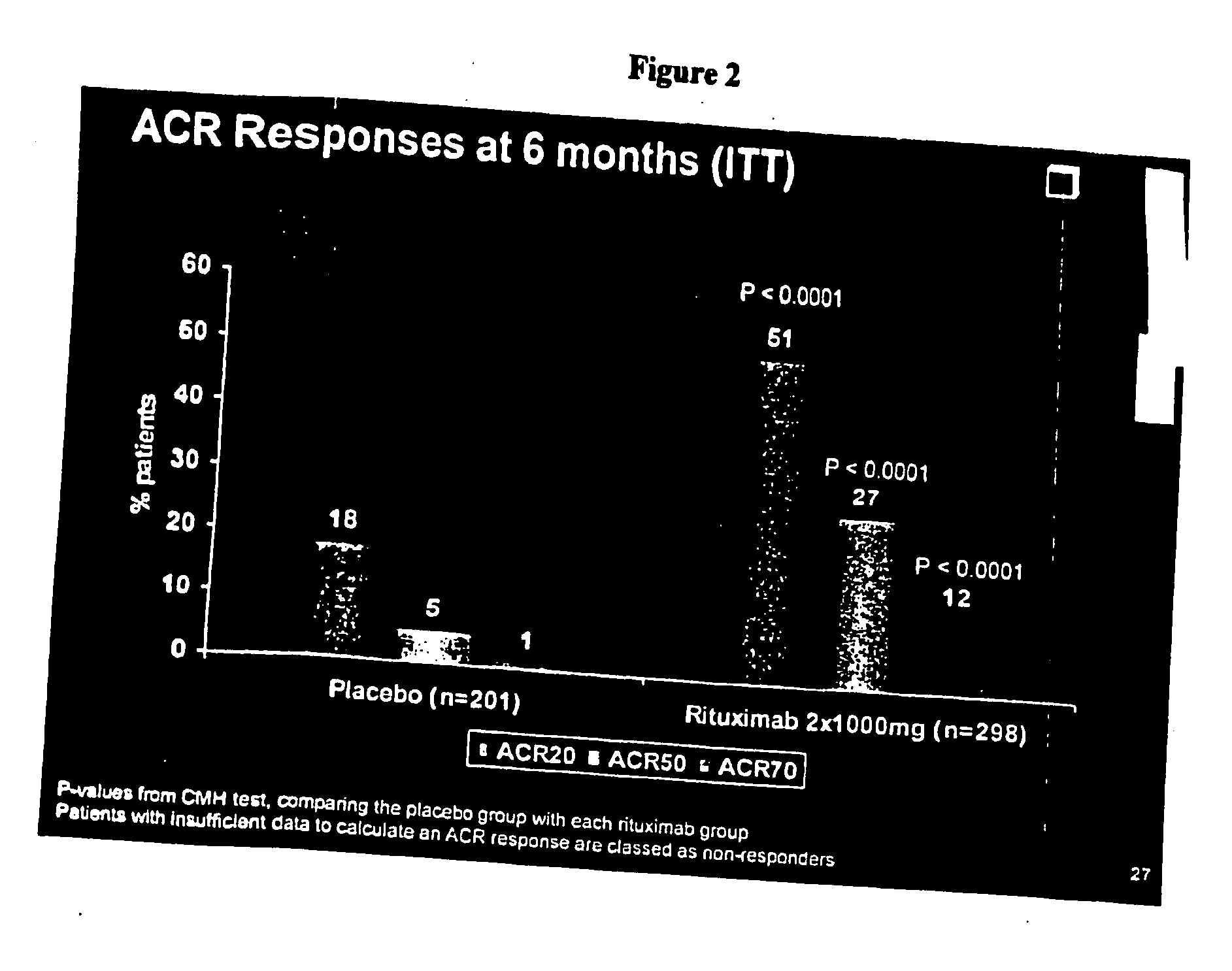
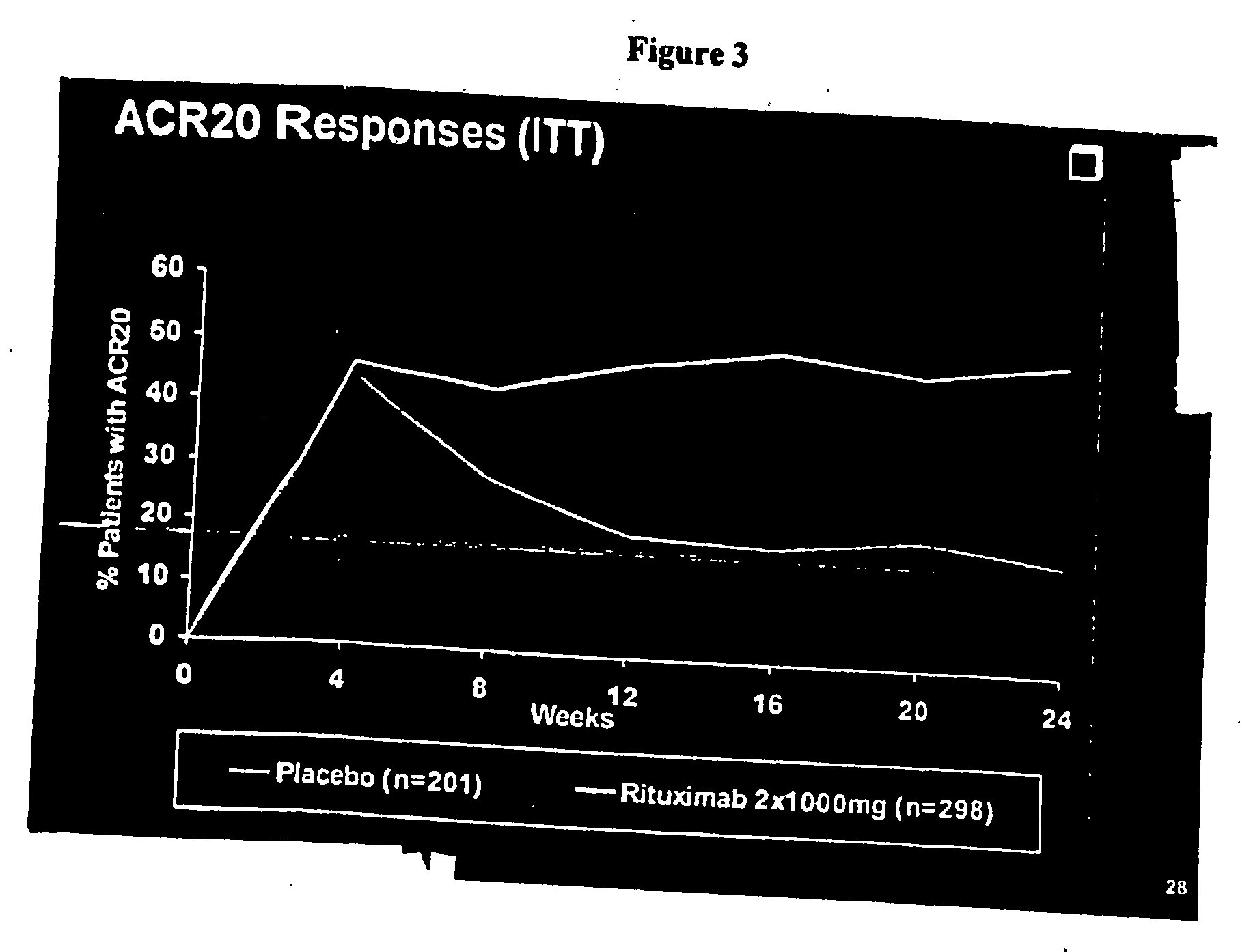
The outcomes of this study were:[0354]Primary endpoint[0355]The proportion of patients with an ACR20 response at Week 24.[0356]Secondary Endpoints[0357]Proportion of patients with ACR50 and ACR70 responses at Week 2...
example 2
Rituximab in RA: Phase III Program
[0417]An important outcome to assess in RA includes the inhibition of progression of structural joint damage and the improvement in physical function. This outcome is particularly important for patients who have recently been diagnosed with RA, since early impact on these has potentially higher long-term benefit. Genovese et al., Arthritis Rheum. 46: 1443-1450 (2002). A population of early RA patients would therefore be an appropriate population to study for these important outcomes.
[0418]With regard to a suitable control treatment for these patients, the current gold standard therapy for early RA is MTX. Consequently, selecting a population of MTX-naïve patients and comparing rituximab plus MTX to MTX alone provides a comparison of this new treatment approach against the current gold standard for these patients.
[0419]This study is a randomized Phase III controlled soluble-blind, parallel-group multicenter study to evaluate the safety and efficacy o...
example 3
Study of Efficacy of Retreatment with Rituximab in Patients with Rheumatoid Arthritis
[0579]This example describes a phase III randomized double-blind, placebo-controlled multicenter study of retreatment with rituximab in subjects with RA receiving background methotrexate.
[0580]The primary objective of this study is to evaluate the efficacy of retreatment with rituximab in subjects with active RA who are receiving MTX and who have had an inadequate response to TNF inhibitors.
[0581]The secondary objectives of this study are as follows:[0582]To evaluate the safety of retreatment with rituximab in subjects with active RA who are receiving MTX and who have had an inadequate response to TNF inhibitors[0583]To evaluate the safety of rituximab in subjects with active RA who are receiving MTX and who have had an inadequate response to TNF inhibitors
[0584]This is a Phase III, randomized, double-blind, placebo-controlled, multicenter study evaluating the efficacy of retreatment with rituximab ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com