Pharmaceutical Compositions for the Treatment of Inner Ear Disorders
a technology for inner ear disorders and compositions, applied in the field of pharmaceutical compositions for the treatment of inner ear disorders, can solve the problems of not being able to meet the needs of patients, not being able to develop effective treatments, and carrying the risk of potent side effects on the central or peripheral nervous system, so as to enhance the diffusion of pharmacologically active agents, suppress or reduce the perception of tinnitus, and increase the permeability of the target middle-inner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods and Materials
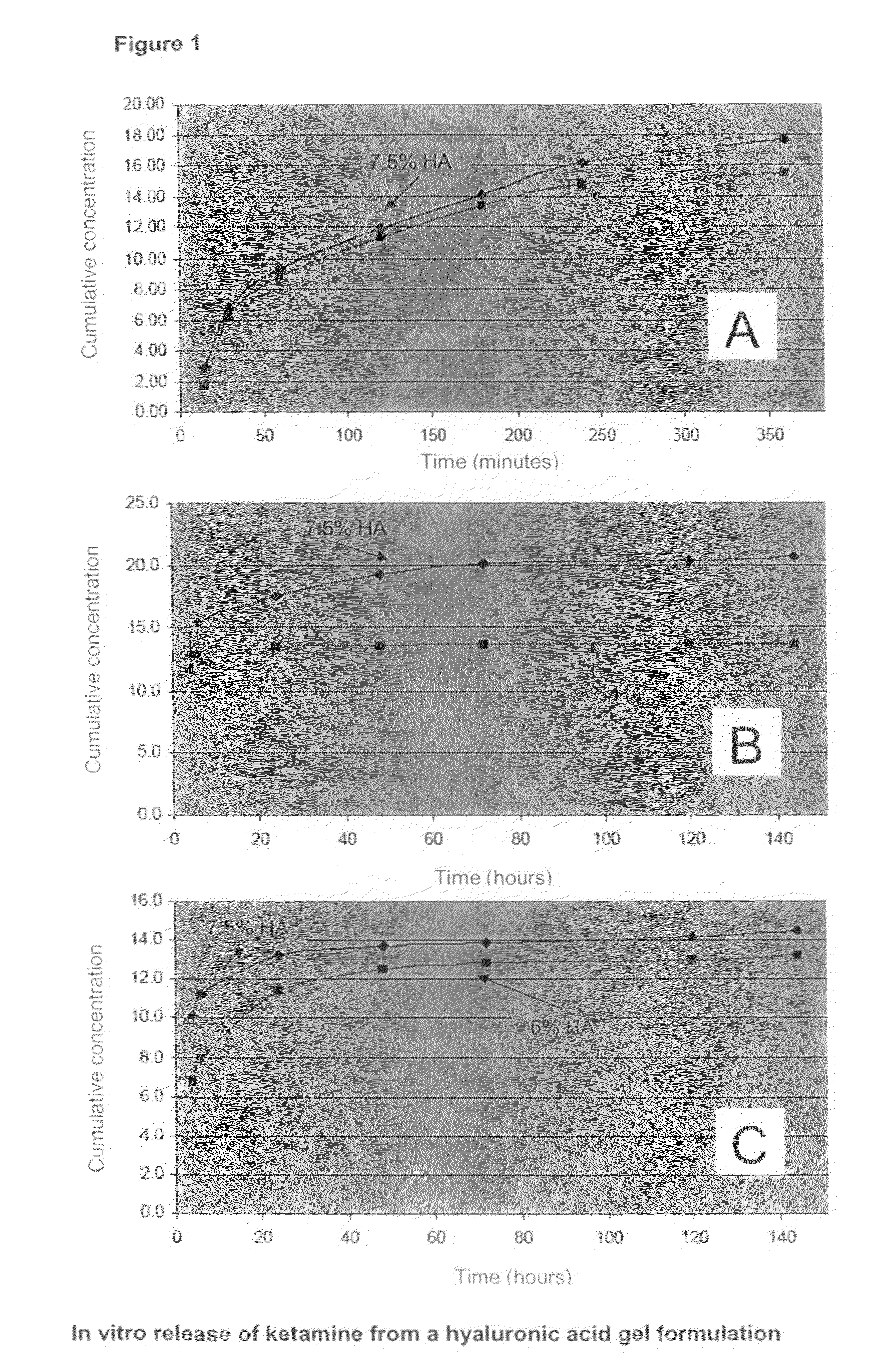
[0080]The release of the NMDA receptor antagonist Ketamine, which had been previously shown to be effective in the treatment of cochlear tinnitus, from a hyaluronic acid gel formulation was evaluated in a two staged approach. In a first stage, in vitro experiments were performed to determine the release kinetics of the formulation. These results were then used as starting point for in vivo studies in animals.
In Vitro Studies
[0081]A hyaluronic acid solution (Hylumed, Genzyme Corp.) was prepared at concentrations of 5 and 7.5% in phosphate buffered saline (PBS). At 8%, handling of the gel had shown to be difficult due to the high viscosity. S-(+)-Ketamine hydrochloride (Sigma-Aldrich) was dissolved at a concentration of 2% (weight / weight) equivalent to 73 mM. To evaluate the importance of the drug load factor, concentrations of 0.5% and 2.5% were also tested. Release of the pharmacologically active agent was measured in PBS, a common receiver fluid for controlled ...
example 2
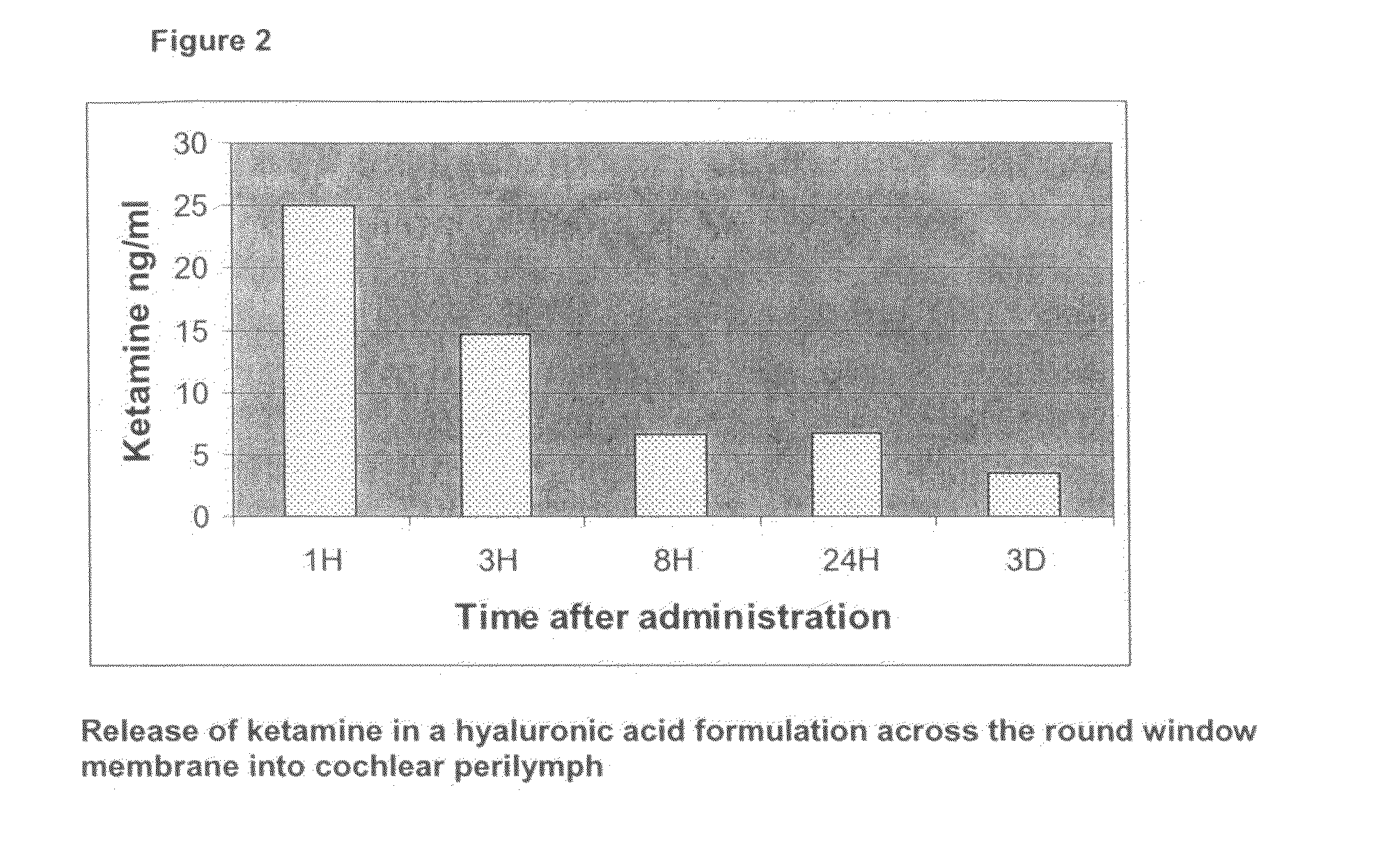
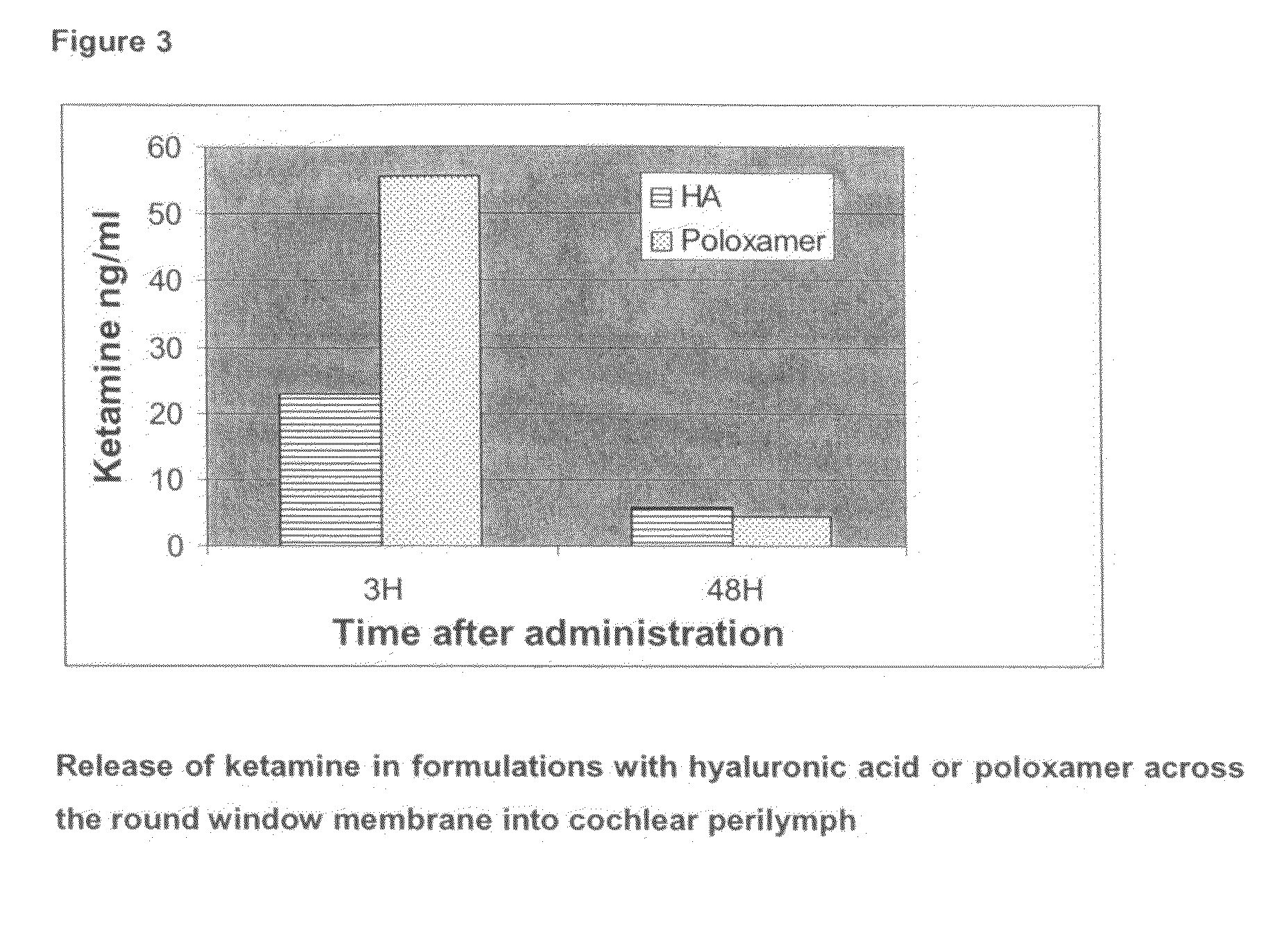
[0092]While the previous experiments explored release kinetics of Ketamine from a rather viscous gel formulation, which could not be injected into the middle ear, we sought next to evaluate two injectable gel formulations, which offer the advantage of easy handling.
Methods and Materials
[0093]A total of 16 pigmented guinea pigs were administered 100 microlitres of either a hyaluronic acid (Hylumed Sterile, Genzyme Corp.) or a poloxamer (Lutrol F127, BASF) gel formulation containing S-(+)-Ketamine hydrochloride (Cristalia) through a 1 ml syringe connected to a needle. Half of the animals received 0.5% hyaluronic acid gel prepared in a phosphate buffered solution at pH 7.4 prepared in accordance with the European Pharmacopeia (ref. 4005000). The Ketamine was dissolved in the gel at a concentration of 1 mM with a magnetic stirrer over night at 4 degrees Celcius. The remaining half of the animals received a 20% poloxamer gel also through a 1 ml syringe connected to a needle. The gel was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com