Electroplating method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1 through 3
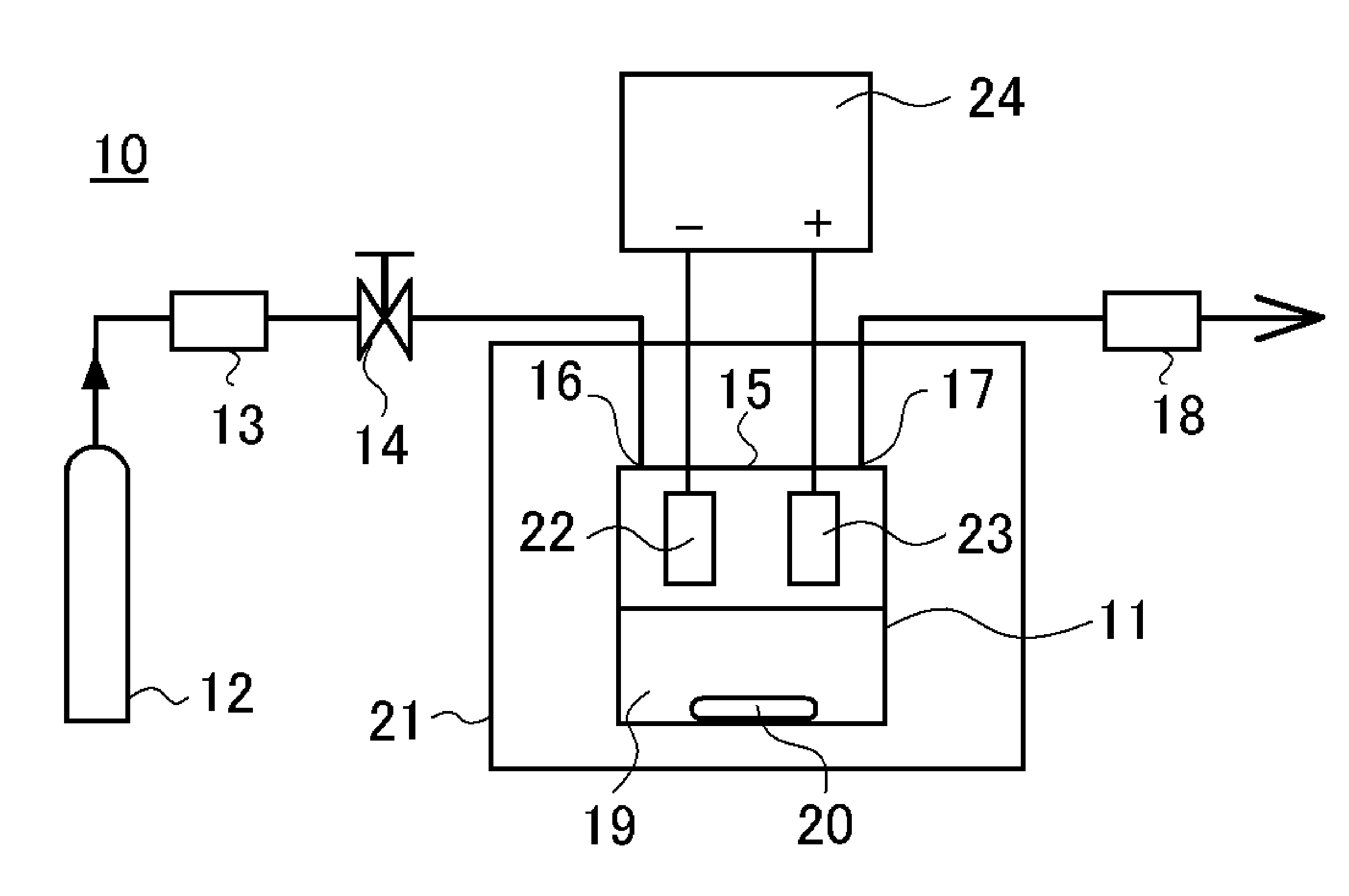
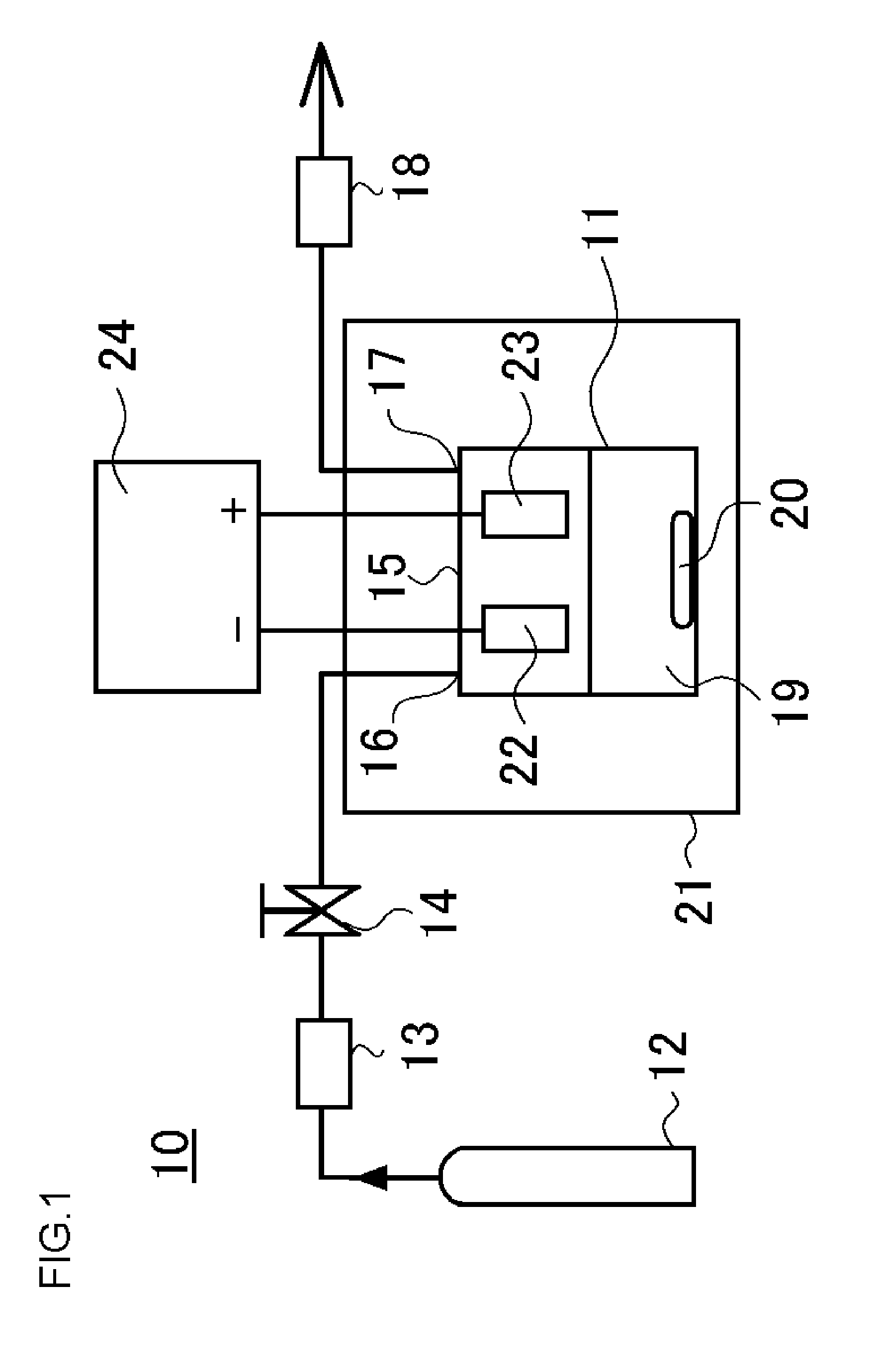
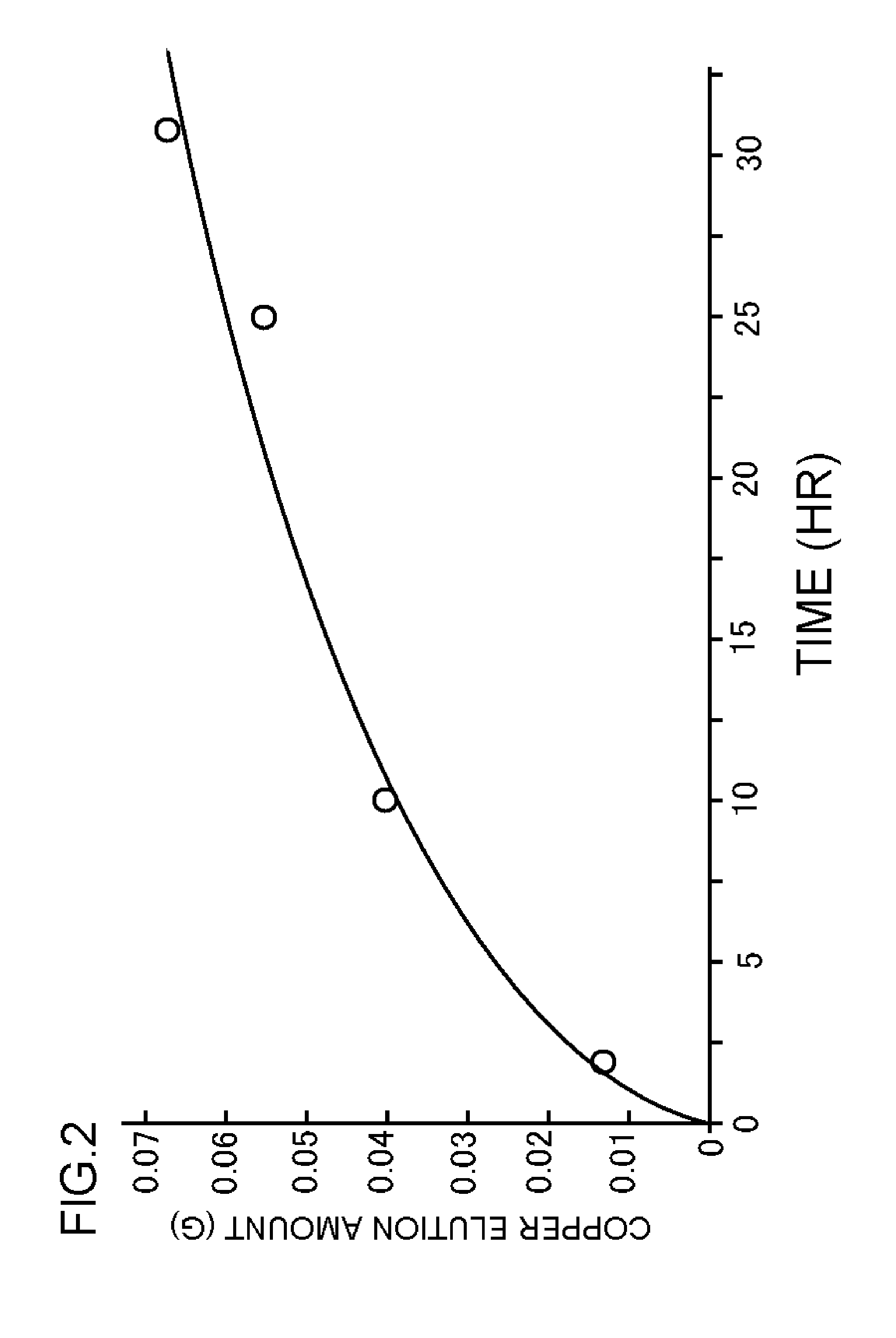
[0055]In Experimental Examples 1 through 3, the solubility of the metal base was investigated when electroplating was conducted in a supercritical state using the high-throw bath. A base obtained by forming a thin copper film having a thickness of 65 nm on a TaN film by sputtering was used as the metal base sample 22. This metal base sample 22 was pretreated by acid pickling and placed together with the counter electrode 23 in the upper part of the electroplating solution 19 in the pressure-resistant plating chamber 11 to as not to be in contact with the electroplating solution 19. The amount of the electroplating solution 19 used was set at 30 mL.
[0056]In this state, the electroplating solution in the pressure-resistant electroplating chamber 11 was heated to a temperature of 40° C.; the electroplating solution 19 was not stirred by the stirrer 20; and the carbon dioxide cylinder 12, the high-pressure pump unit 13, the valve 14, and the pressure adjustment unit 18 were operated to ...
experimental example 1
[0059]Five minutes after the pressure inside the pressure-resistant electroplating chamber 11 became 10 MPa, an electroplating voltage was applied between the metal base sample 22 and the counter electrode 23 from the direct current power source 24.
experimental example 2
[0060]At the same time the pressure inside the pressure-resistant electroplating chamber 11 became 10 MPa, an electroplating voltage was applied between the metal base sample 22 and the counter electrode 23 from the direct current power source 24.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle diameter | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
| Particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com