Anti-freeze protein enhanced nucleic acid amplification
a nucleic acid amplification and anti-freeze technology, applied in the field of anti-freeze protein can solve the problems of particularly problematic non-specific amplification, achieve enhanced nucleic acid amplification, improve enzyme stability, and improve the performance of nucleic acid amplification reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Anti-Freeze Proteins (AFPs) Enhance Stability of Real Time-PCR Master Mix During Extended Periods of Storage at −20°
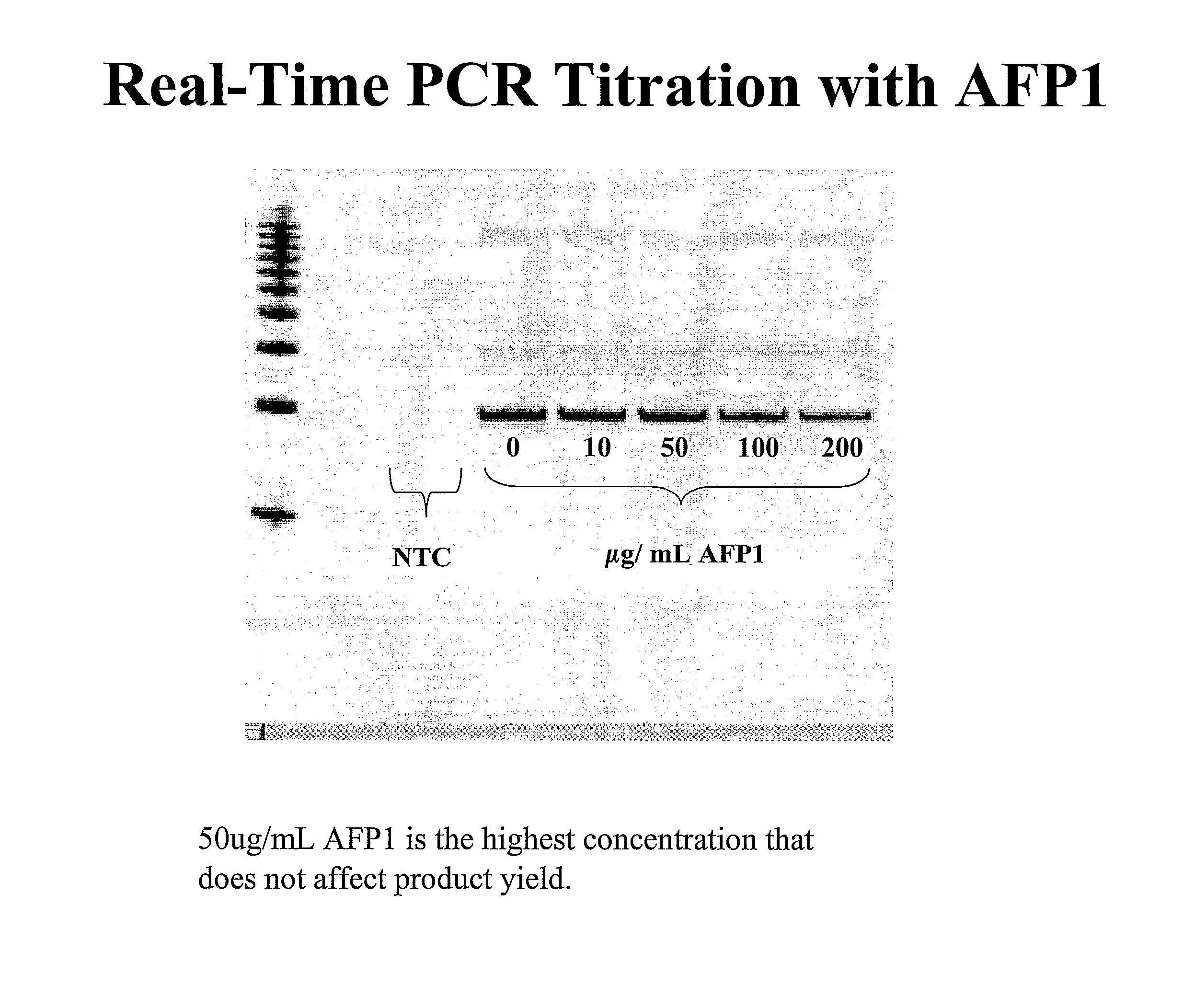
[0087]The effects of AFP1 on the freeze / thaw stability of real-time PCR buffers were investigated. Initially, AFP1 was titrated into real time-PCR reactions to determine what concentration of AFP1 did not detrimentally affect amplicon yield. Real-time PCR was performed as described in Eppendorf RealMasterMix Probe + / − ROX (catalog #00320900.525), and products visualized on ethidium bromide stained 1% agarose gels. As shown in FIG. 1, product yields were not detrimentally affected until approximately 100 μg / ml AFP1 was included in the reaction mix. Conversely, the addition of 50 μg / ml AFP1 to the real time-PCR had little or no effect on the reaction yields (compare the lane having 0 AFP1 to the lane having 50 μg / ml AFP1).
[0088]Prior to determining the freeze / thaw capacity of Real-Time PCR buffers in the presence and absence of AFP1, the ability of AFP1 to enhance fresh ...
example 2
pH Modification and Buffer Type have Significant Effect on Optimizing Sorbitol, AFP1 Containing Real-Time PCR Buffer
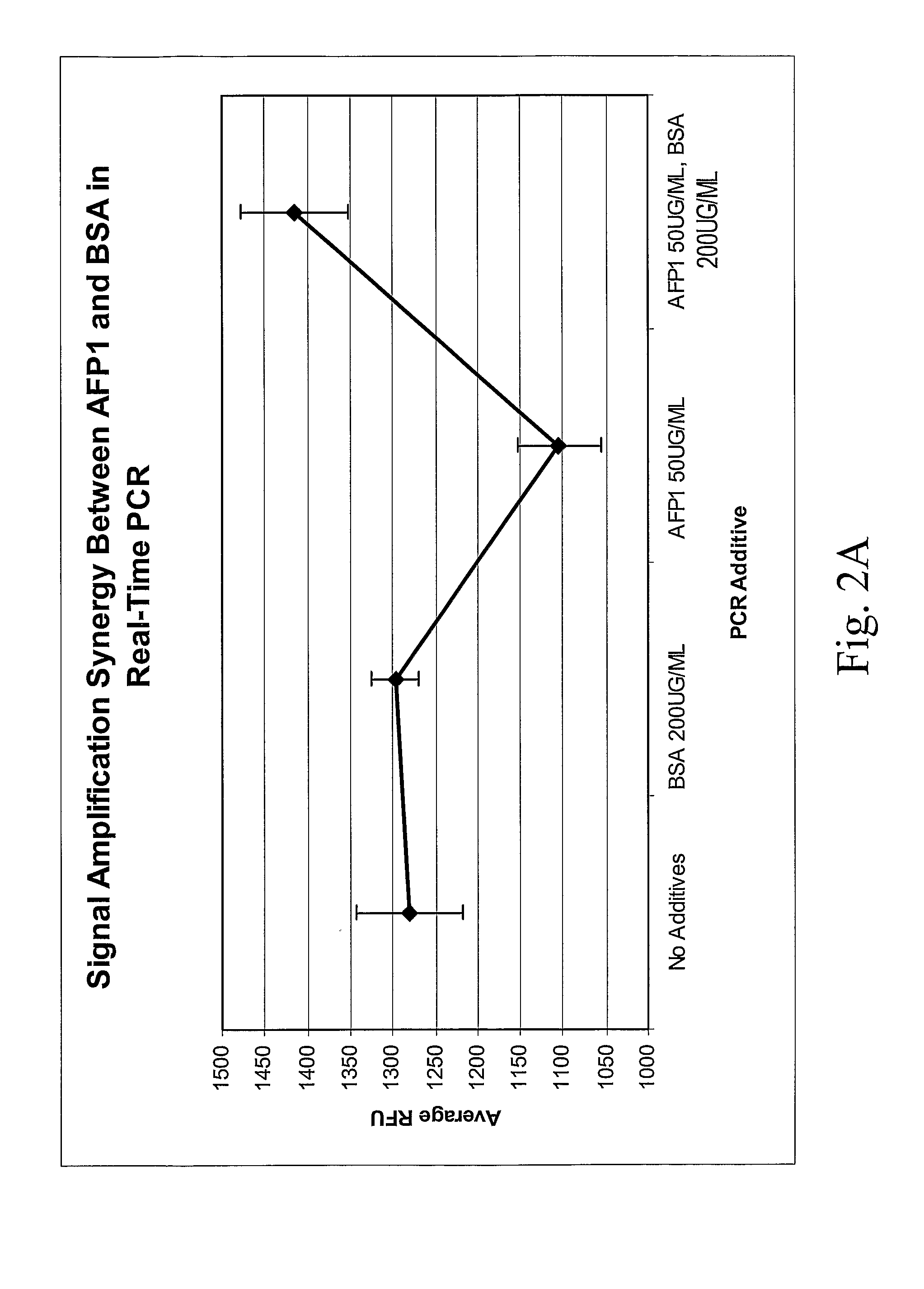
[0093]Several real time-PCR buffer combinations were tested for signal amplification and for threshold cycle in the presence of a standard amount of both sorbitol and AFP1 / BSA. Both the buffering component and salt were modified to provide different ionic concentrations and pHs. In particular, 25 mM HEPES-KOH with 15 mM KCL pH 8.0; 25 mM TAPS-Tris with 15 mM K GLUTpH 8.0; 25 mM HEPES-Tris with 50 mM KCL pH 8.0; 25 mM Bicine-Tris with 15 mM K GLUT pH 8.7; 25 mM HEPES-KOH with 15 mM K GLUTpH 8.0; 25 mM Bicine-Tris with 50 mM K GLUT pH 8.0; 25 mM TAPS-KOH with 15 mM KCL pH 8.0; 25 mM Bicine-Tris with 15 mM KCL pH 8.0; 25 mM TAPS-Tris with 50 mM KCL pH 8.0; and 25 mM Bicine-Tris with 50 mM KCl pH 8.4 were compared for ability to support highly accurate real time-PCR (each buffer also included 50 μg / ml AFP1, 200 μg / ml BSA and 100 mM sorbitol).
[0094]As shown graphically in F...
example 3
AFP Containing PCR Master Mix Functionally Outperforms Competitor PCR Mix
[0097]An embodiment of a PCR Master Mix prepared in accordance with the present invention was functionally compared to Invitrogen Platinum qPCR Supermix-UDG for its ability to support long term stability within real time-PCR. Long term stability test experimental parameters are shown in Table 5. Cycling parameters included: 95° C. for one minute and forty cycles of 95° C. for twenty seconds, 56° C. for ten seconds and 68° C. for thirty seconds.
TABLE 5Long Term Stability TestComponentStockFinalμl / 50 μl rxn3.5Aliquot 70 μl of each of the RealMaster, 2.5X master mix into a biopure tube2.5X Real Master2.512070Mix + / − ROX(x)Primer-Probe Template Mix For 2.5X MMB2M fwd primer100.2122(μM)B2M rev primer100.2122(μM)B2M FAM probe7.50.15122(μM)gDNA male501122(50 ng / μl)MBGW26572Total30660Add 105 μl (3.5 rxn) of primer-probe-template mix to each aliquot of 2.5X master mix.Aliquot 87.5 μl of each of the competitors 2X master...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com