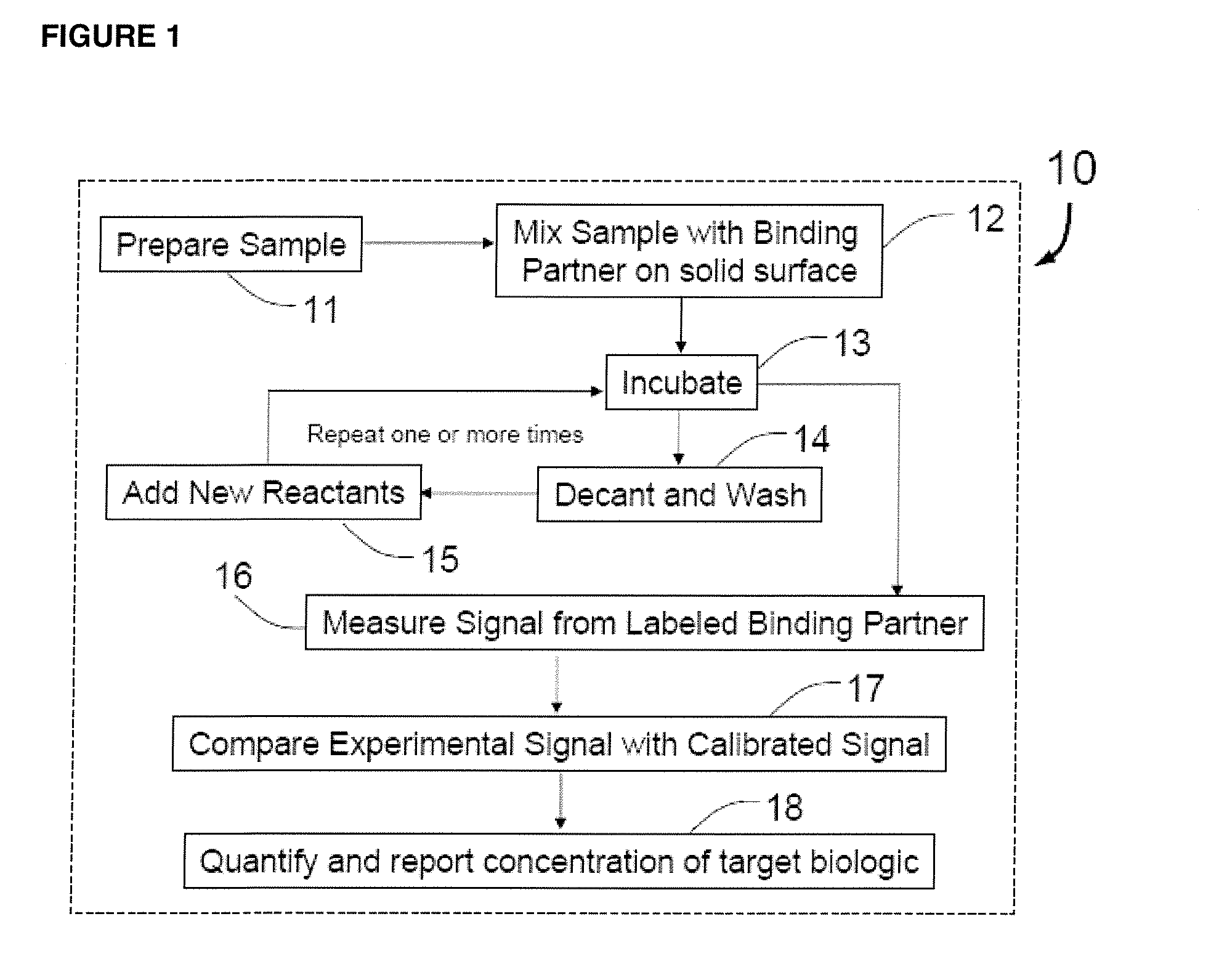
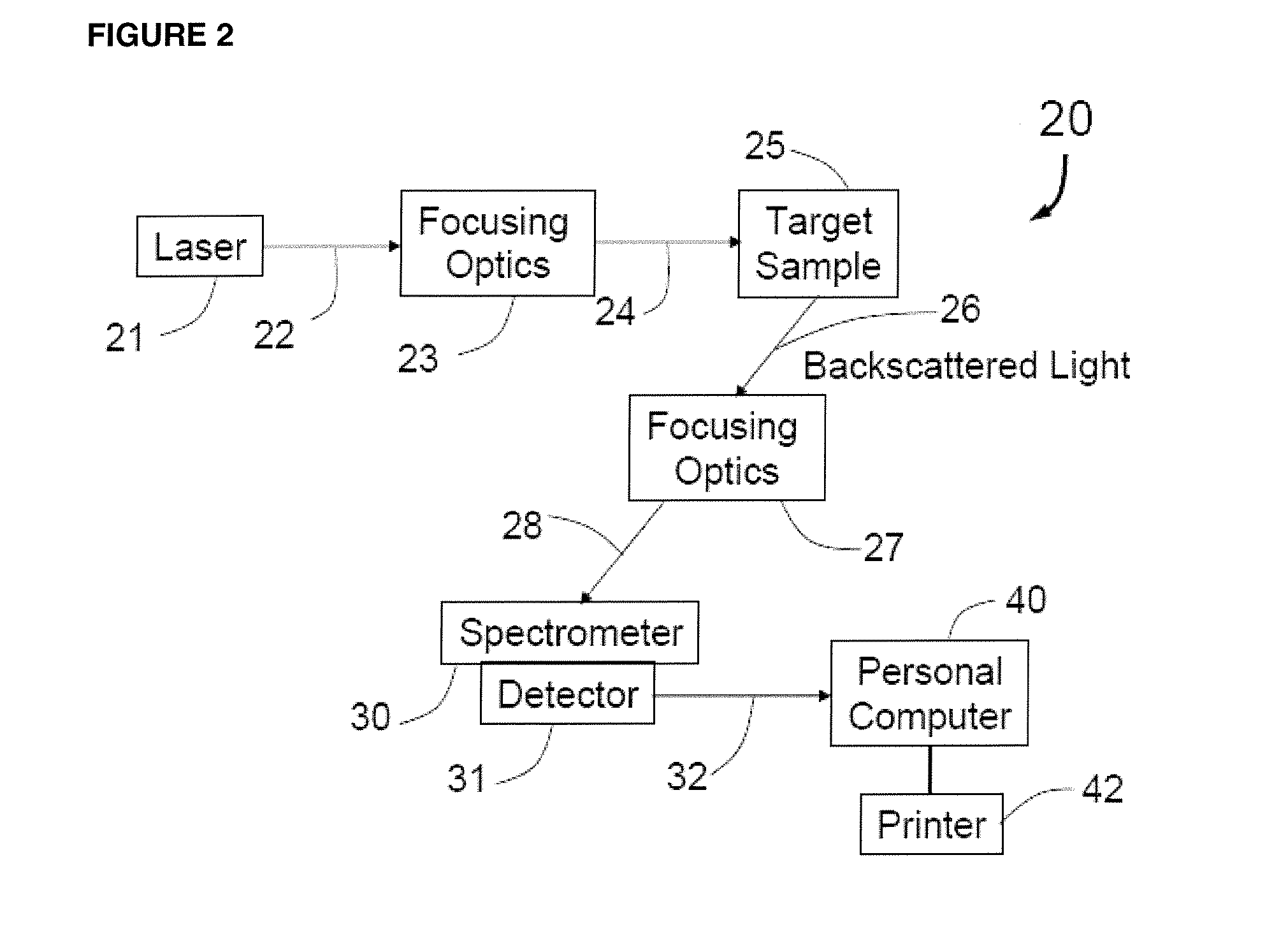
Methods for detecting organisms and enzymatic reactions using raman spectroscopy and aromatic compounds comprising phosphate
a technology of raman spectroscopy and aromatic compounds, applied in the field of biological diagnostic equipment and testing methods, can solve the problems of difficult detection of antibodies, limited accuracy, specificity and sensitivity of methods and techniques, and often extensive sample preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Detection of Listeria Using BASH-UP
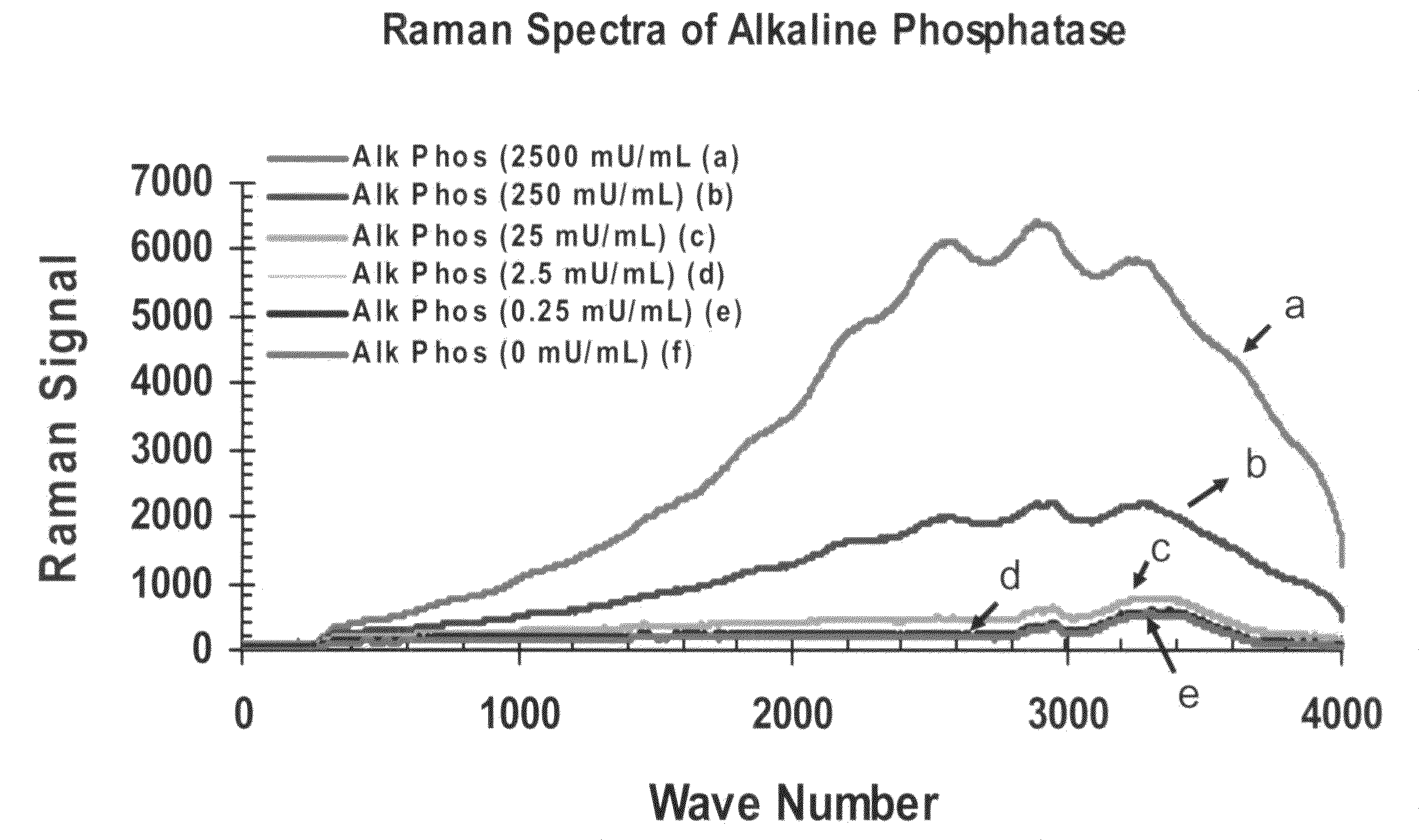
[0313]FIG. 8 depicts Raman spectra obtained from an enzyme-linked immunoassay for the pathogenic bacteria Listeria utilizing the two-component BASH-UP chemistry, an enzyme-linked antibody, and Raman detection procedure described below utilizing the following buffers and reagents:
[0314]Working Saline Buffer (Used for Washes in Protocol):
[0315]10 mM Sodium Phosphate, pH 6.0
[0317]2.67 mM Potassium Chloride
[0318]0.09 mM Ethylenediaminetetraacetic acid (EDTA)
[0319]0.05% Bronidox-L
[0320]Final Chemistry Reagent (BASH):
[0321]0.588 mM 5-Aminosalicylic Acid
[0322]0.145 mM 2-Hydroxybenzyl alcohol
[0323]0.005 mM L-Ascorbic Acid
[0324]0.09% Tween-20
[0325]UP Component:
[0327]Working Saline Buffer
[0328]Additional Reagents:[0329]1. Microparticles—Anti-Listeria (antibody) coated magnetic microparticles at 2 million microparticles / sample upon addition.[0330]2. Conjugate Solution—Anti-Listeria (antibody) conjugated ...
example 2
Colorimetric Assays of Horseradish Peroxidase (HRPO)
[0353]Colorimetric assays of Horseradish Peroxidase (HRPO) activity were conducted to obtain data that could be compared with the Raman-based methods. TMB develops a deep blue soluble product when reacted with horseradish peroxidase. ABTS develops a blue-green product when reacted with horseradish peroxidase.
[0354]Colorimetric assays were performed with the TMB and ABTS reactions using two different methods:
[0355]Method A (TMB): HRPO dilutions were made to measure 1000 pg to 0.0125 pg per 50 μl sample in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) at pH 7.4. 50 μl HRPO sample per dilution was added to 200 μl TMB reagent and allowed to react for 15 or 30 minutes at which time 200 μl stop-solution (KPL Laboratories) was added. Absorbance was measured at 450 nm for each sample.
[0356]Method B (ABTS): HRPO dilutions were made to allow 1000 pg to 0.0125 pg per 50 μl sample in PBS at pH 7.4. 50 μl HRPO sampl...
example 3
Fluorogenic and Chemiluminescent Assays of HRPO
[0358]Several reagents were tested: Sigma Chemiluminescent Peroxidase Substrate, Pierce Fluorogenic (Chemifluorescent) Substrate Kit, AnaSpec Sensolyte ADHP Fluorogenic Substrate, Invitrogen Molecular Probes Amplex Red Fluorogenic Substrate, and KPL Laboratories LumiGLO. Sigma and Pierce substrates did not work with HRPO in PBS or with BSA-containing buffer.
[0359]A. AnaSpec Fluorogenic ADHP Assay
[0360]AnaSpec Fluorogenic kit utilizes ADHP (10-acetyl-3,7-dihydroxyphenoxazine) to analyze peroxidase in solution whereby ADHP is oxidized in the presence of peroxidase and hydrogen peroxide. The oxidized product of ADHP (resozufin) gives pink fluorescence that can be measured at the emission wavelength of 590 nm with the excitation wavelength of 530-560 nm. An overdose of peroxidase in the assay will further convert the fluorescent resorufin to non-fluorescent resozurin to yield reduced fluorescent signal. HRPO dilutions were made to allow det...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com