High strength member for intracorporeal use
a high-strength, intracorporeal technology, applied in the field of intravascular members, can solve problems such as specimens, achieve the effects of maintaining compliance, facilitating insertion, and minimizing the alteration of natural physiology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
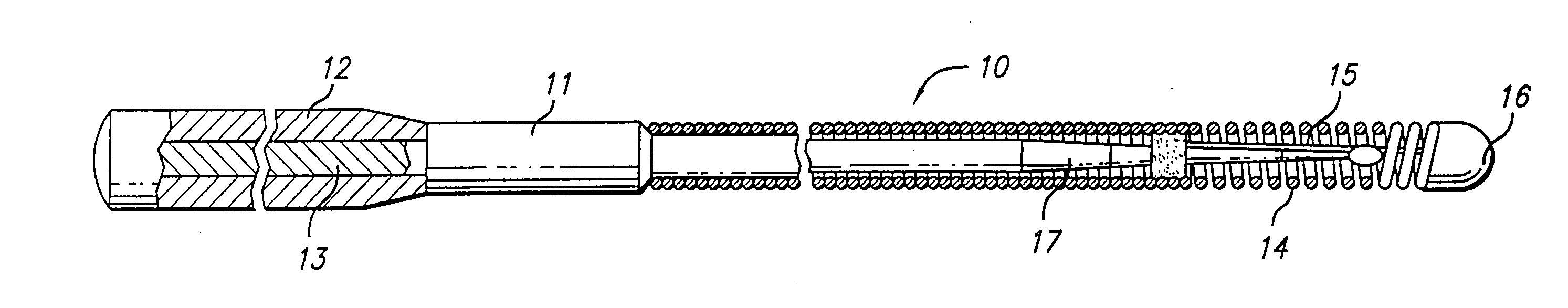
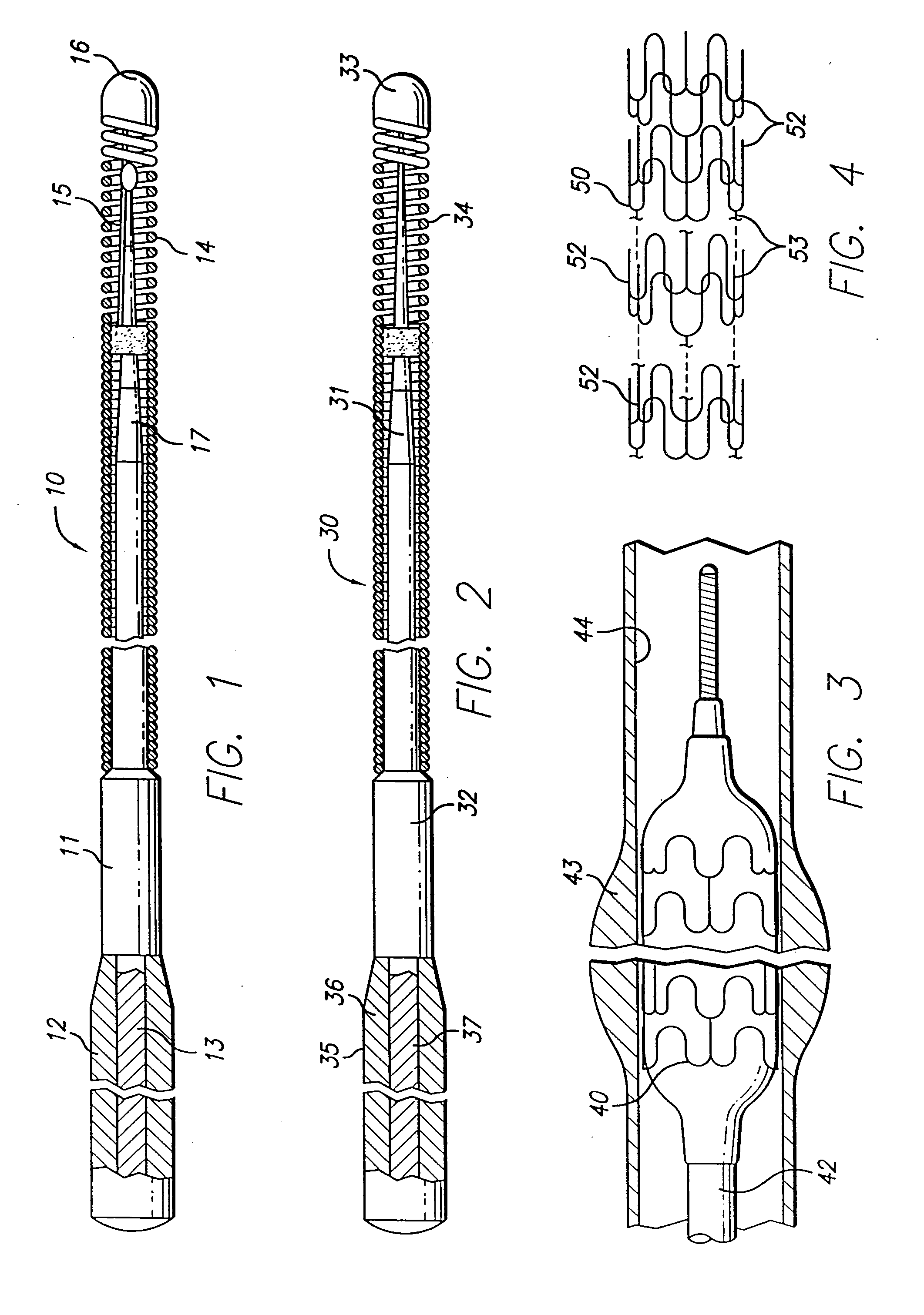
[0038]FIG. 1 illustrates a guidewire 10 which includes a core member 11 having an outer sheath 12 formed of a Co—Ni—Cr alloy and an inner member 13 formed of Ni—Ti, a helical coil 14 on the distal end of the core member with a shaping ribbon 15 extending between the distal end of the core member and a rounded plug 16 which connects the distal end of the shaping ribbon with the distal end of the helical coil. The distal section 17 of the core member 11, which is disposed primarily within the coil 14, is tapered to sequentially smaller diameters to provide gradually increasing flexibility along the length of the distal portion of the guidewire 10. The taper is formed by removing the sheath 12 formed of high strength Co—Ni—Cr alloy which exposes the inner NiTi alloy member 13 having moderate strength and substantial flexibility which may then be ground in a conventional manner to one or more smaller diameter sections.
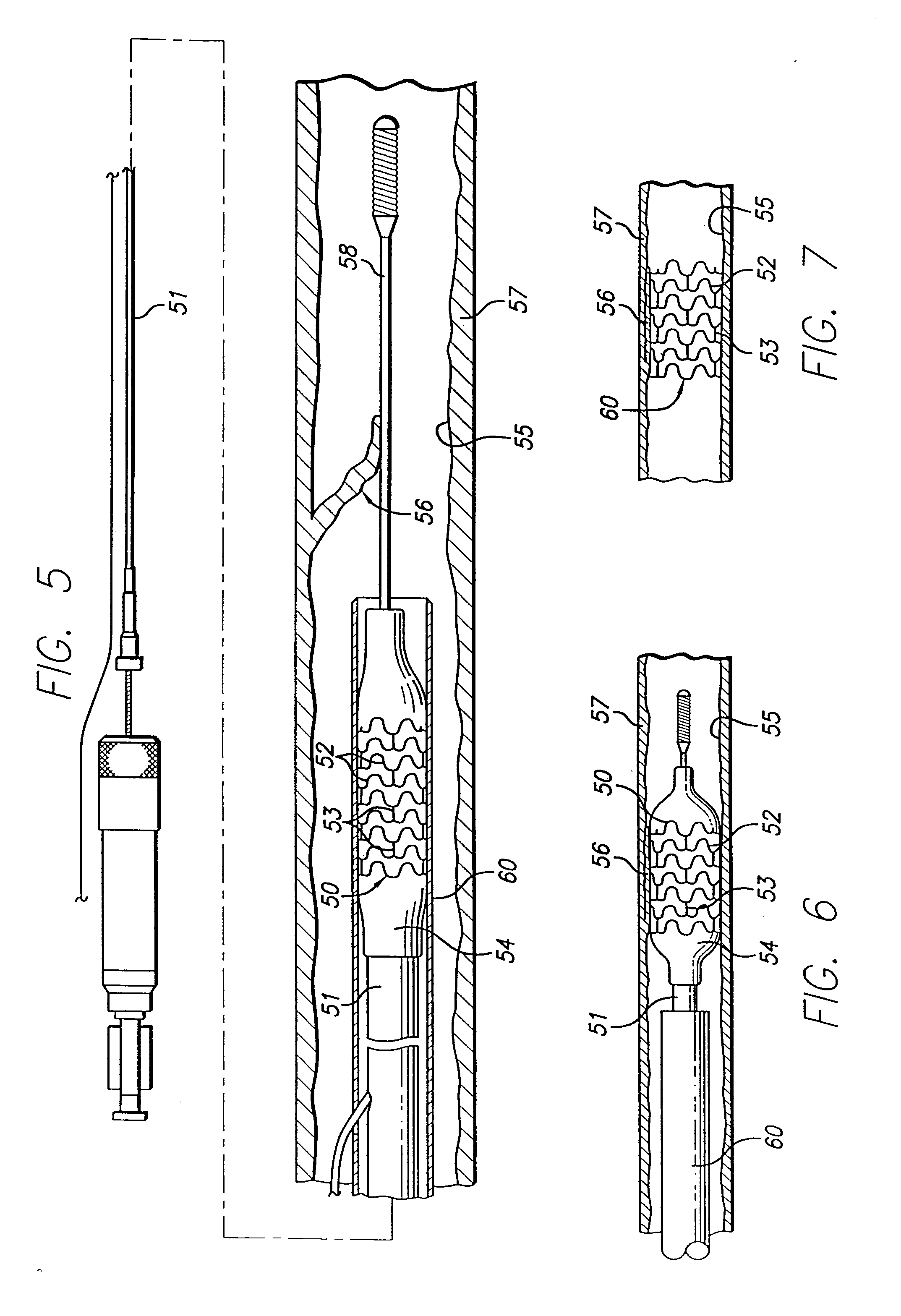
[0039]FIG. 2 depicts a guidewire 30 with a construction wherein the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| height-to-width aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com