Biochip assembly and assay method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
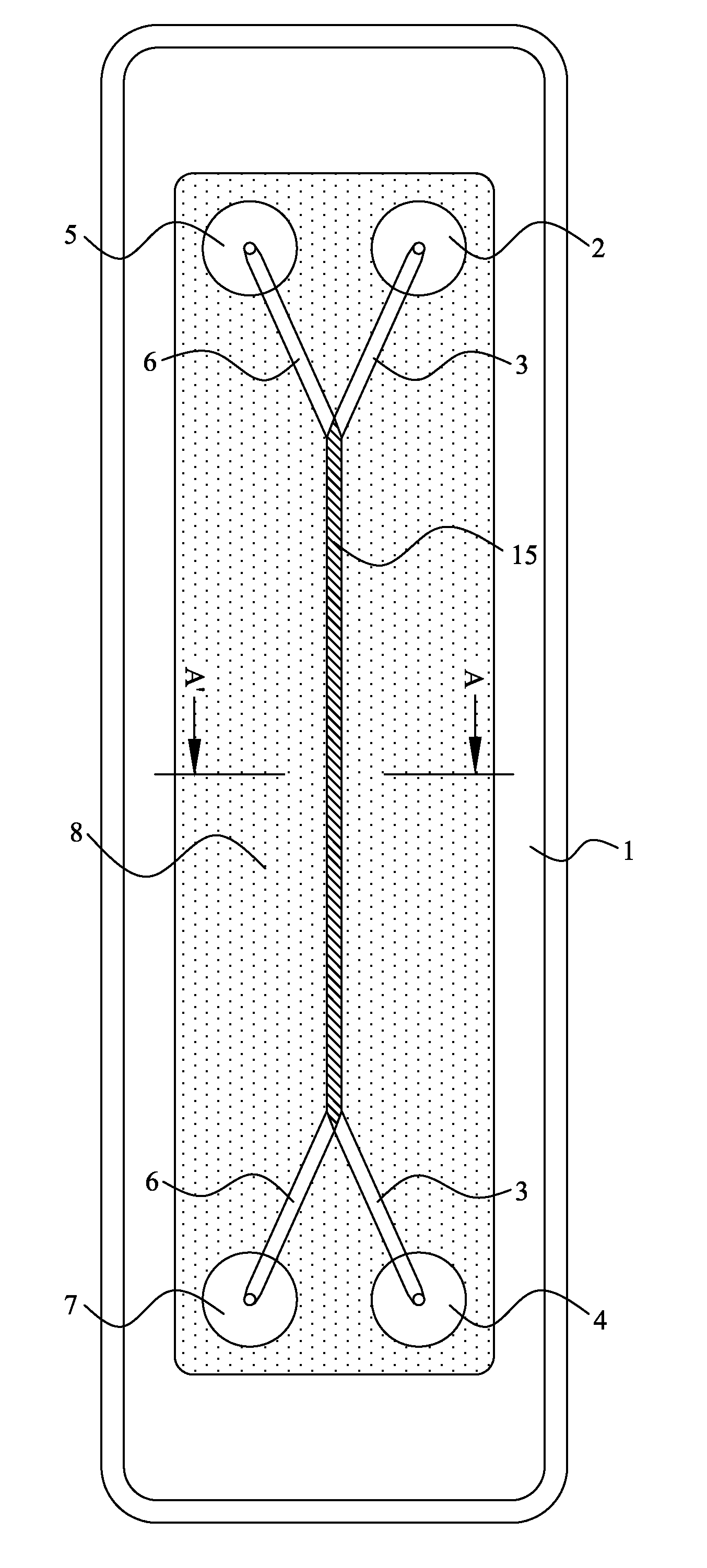
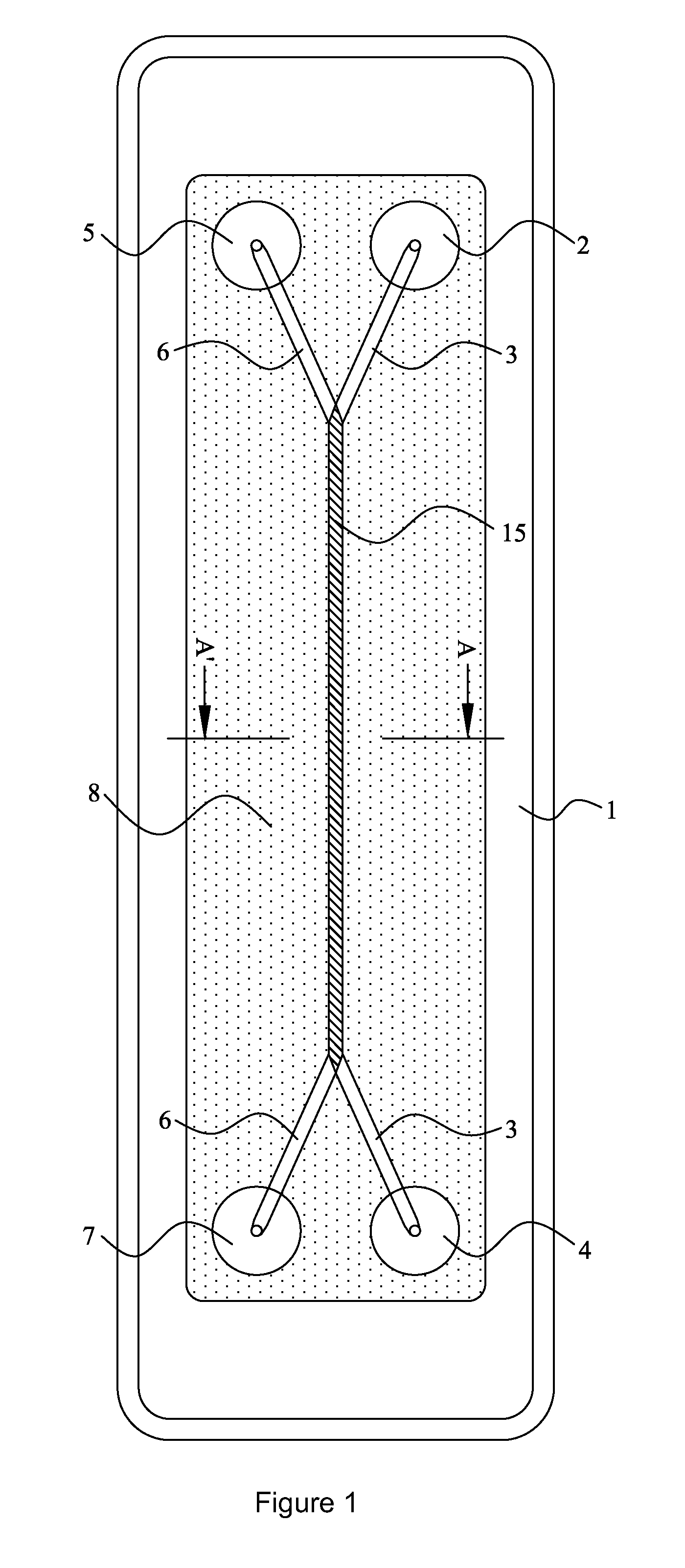
[0097]The invention provides a device and method for performing cell-based assays and cell tests. Prior to discussing this invention and figures in further detail, the following terms used in the specification will first be explained.
[0098]The term “cell” includes both eukaryotic and prokaryotic cells, including but not limited to bacteria, yeast, mammalian cells. The use of plant cells may also be contemplated. Preferably the cells are eukaryotic cells. According to one particularly preferred embodiment the cells are leukocytes, such as neutrophils, lymphocytes etc.
[0099]The term “sample cells” or “sample cell containing liquid” ideally refers to a suspension of living cells within a suitable carrier medium, for example, a culture medium. Such a culture medium is ideally in liquid form but is not limited to this form. It will be understood that more than one type of cell may be in the suspension.
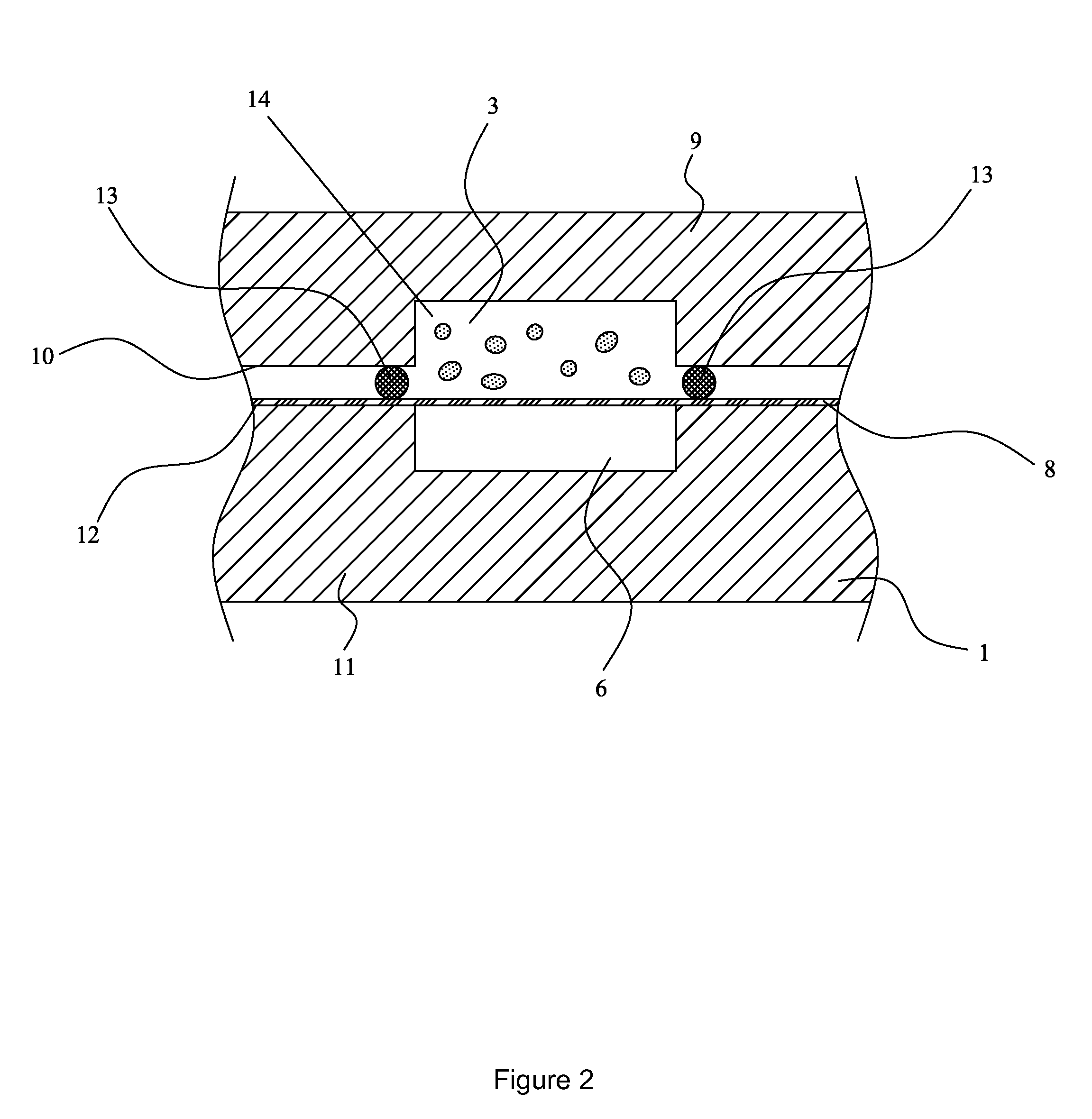
[0100]The semi-permeable membrane may be a cell-transparent membrane. These terms will ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com