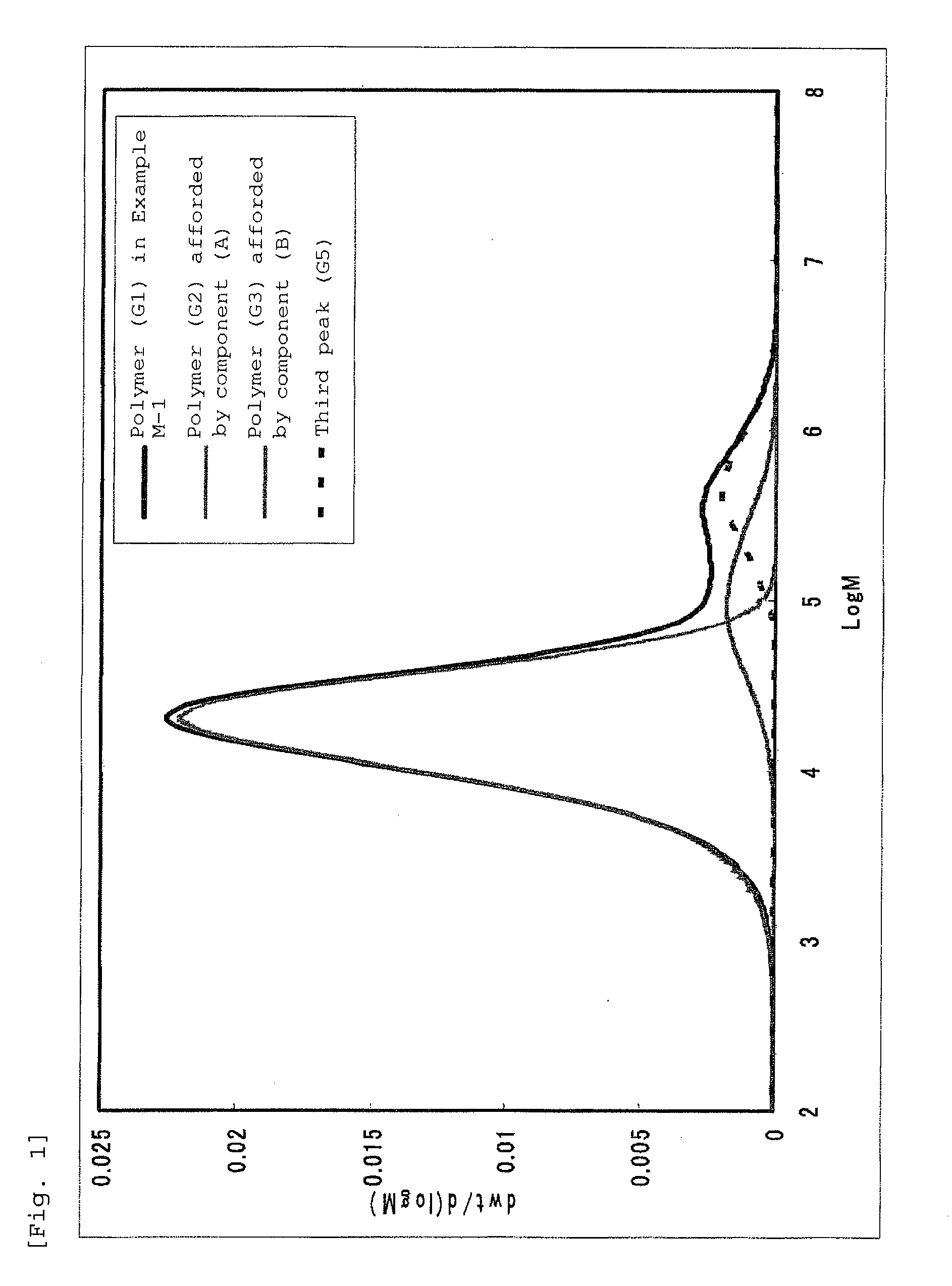
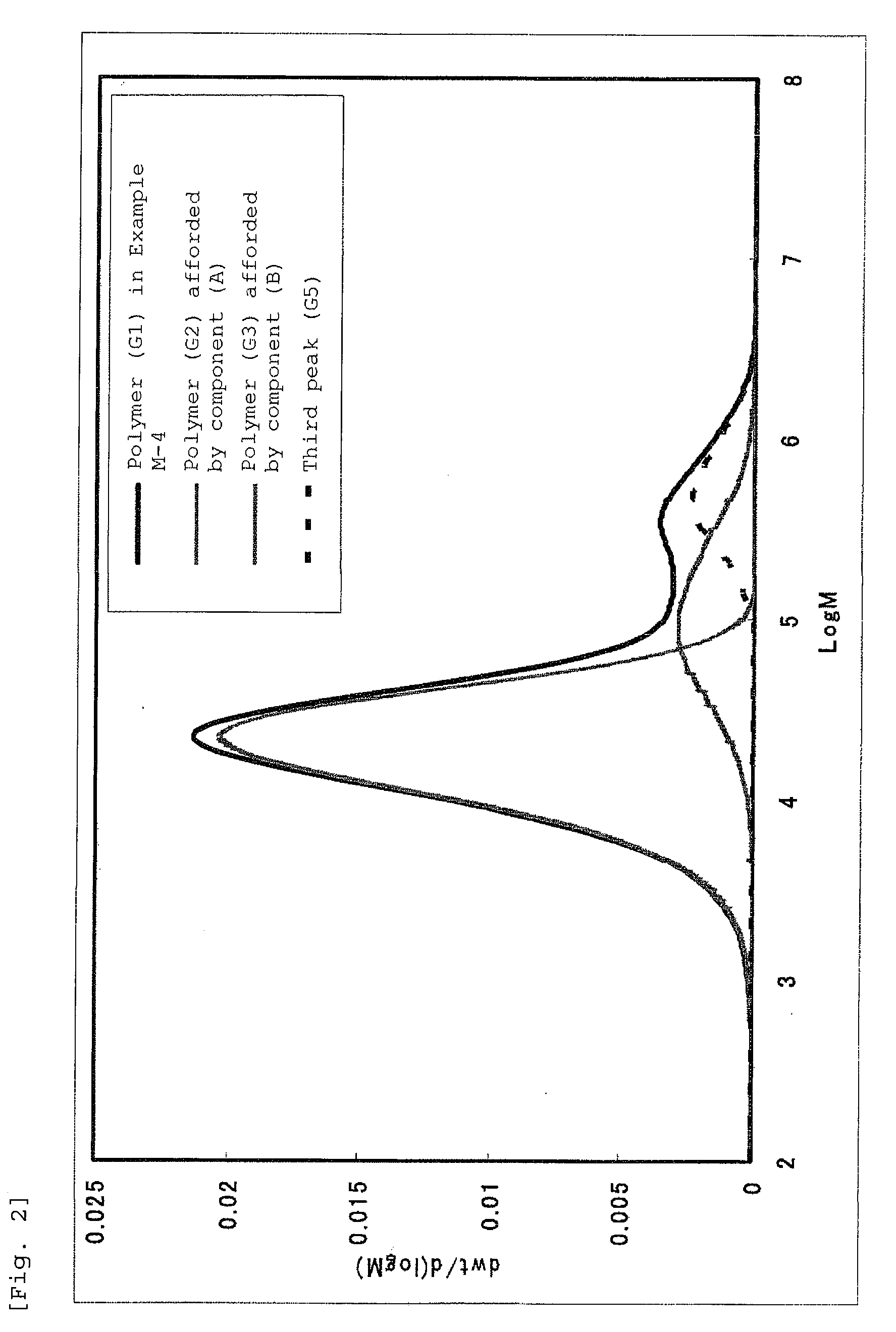
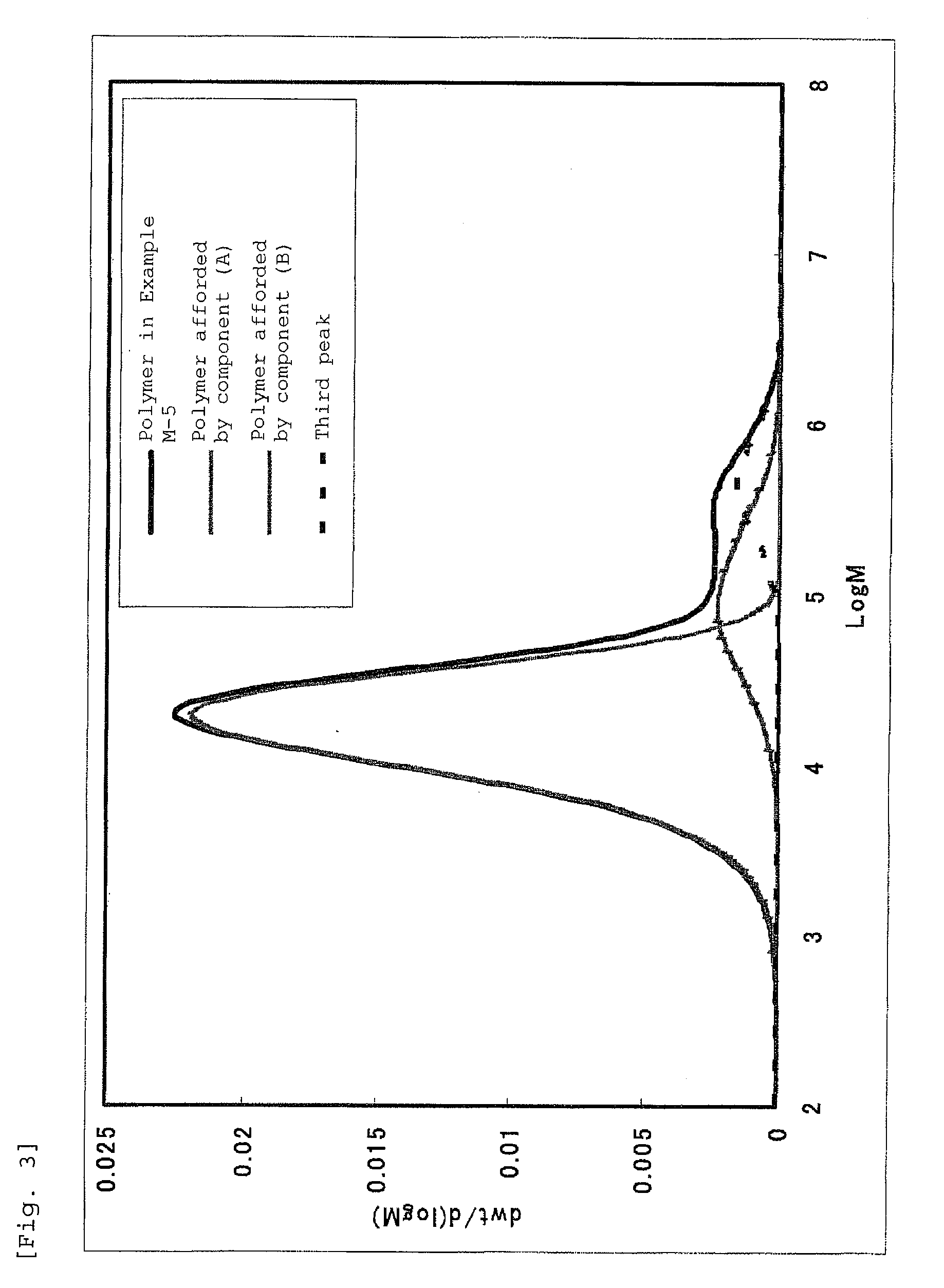
Bridged metallocene compound, olefin polymerization catalyst containing the same, and ethylene polymer obtained with the catalyst
a technology of olefin polymerization and metallocene, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of poor high-speed film-forming properties of polymers, low mechanical strength properties of shaped articles, and low tensile strength and impact resistance strength, etc., to achieve high-speed film-forming efficiency, increase the number of terminal double bonds, and low molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthetic example 1
Synthesis of dimethylsilylene (cyclopentadienyl) (3-ethylcyclopentadienyl) zirconium dichloride (A1)
Synthesis of chloro(cyclopentadienyl)dimethylsilane
[0467]THF in a volume of 100 ml was added to 14.3 g (110 mmol) of dimethylsilyl dichloride, and the mixture was cooled to −78° C. A 2 M THF solution of sodium cyclopentadiene in a volume of 38.7 ml (77.4 mmol) was added thereto dropwise over a period of 30 minutes, and the temperature was gradually increased. The mixture was stirred at room temperature for 24 hours, and was concentrated under reduced pressure. Insolubles were removed by filtration. The filtrate was washed with hexane, and the hexane was distilled away from the filtrate under reduced pressure, thereby obtaining chloro(cyclopentadienyl)dimethylsilane. The compound was used in the next step.
Synthesis of (3-ethylcyclopentadienyl) (cyclopentadienyl) dimethylsilane
[0468]Ethylcyclopentadiene in an amount of 7.52 g (80 mmol) was dissolved in 100 ml of THF, and the solution ...
synthetic example 2
Synthesis of dimethylsilylene(3-n-propylcyclopentadienyl) (cyclopentadienyl) zirconium dichloride (A2)
Synthesis of chloro(cyclopentadienyl)dimethylsilane
[0471]THF in a volume of 100 ml was added to 14.3 g (110 mmol) of dimethylsilyl dichloride, and the mixture was cooled to −78° C. A 2 M THF solution of sodium cyclopentadiene in a volume of 38.7 ml (77.4 mmol) was added thereto dropwise over a period of 30 minutes, and the temperature was gradually increased. The mixture was stirred at room temperature for 24 hours, and was concentrated under reduced pressure. Sodium chloride was removed by filtration. The filtrate was washed with hexane, and the hexane was distilled away from the filtrate under reduced pressure, thereby obtaining chloro(cyclopentadienyl)dimethylsilane. The compound was used in the next step.
Synthesis of dimethylsilyl (3-n-propylcyclopentadienyl) (cyclopentadienyl)
[0472]THF in a volume of 100 ml was added to 2.16 g (20 mmol) of n-propylcyclopentadiene, and the mix...
synthetic example 3
Synthesis of dimethylsilylene(cyclopentadienyl) (3-n-butylcyclopentadienyl)zirconium dichloride (A3)
Synthesis of (3-n-butylcyclopentadienyl) chlorodimethylsilane
[0476]THF in a volume of 50 ml was added to 30.1 g (61.5 mmol) of a 25 wt % THF solution of butylcyclopentadiene, and the mixture was cooled to 0° C. A 1.52 M hexane solution of n-butyllithium in a volume of 38.4 ml (58.4 mol) was added thereto dropwise. The mixture was stirred at room temperature for 2 hours and was added dropwise to 50 ml of THF containing 14.3 g (110 mmol) of dimethylsilyl dichloride at −78° C. The temperature was gradually increased, and the mixture was stirred at room temperature for 24 hours and was concentrated under reduced pressure. Insolubles were removed by filtration. The filtrate was washed with hexane and was distilled under reduced pressure, thereby obtaining 8.09 g of (3-n-butylcyclopentadienyl)chlorodimethylsilane (yield: 64%). The compound was identified by GC-MS. GC-MS: 214 (MS).
Synthesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow rate | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com