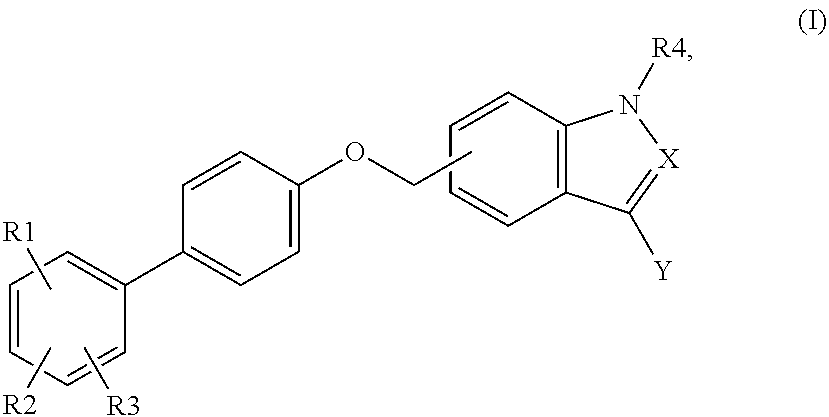
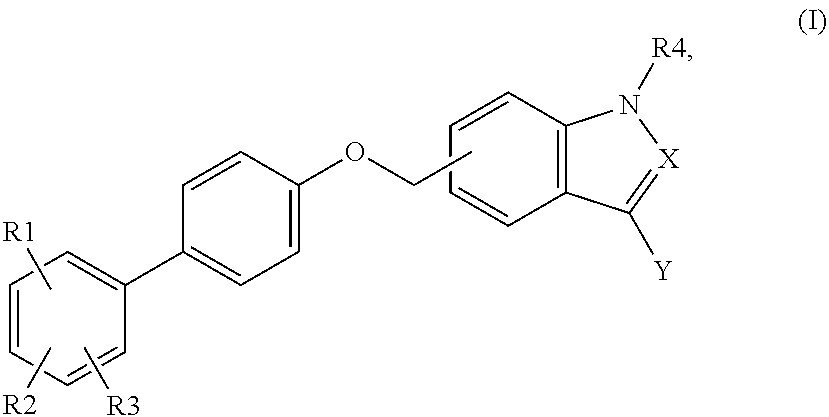
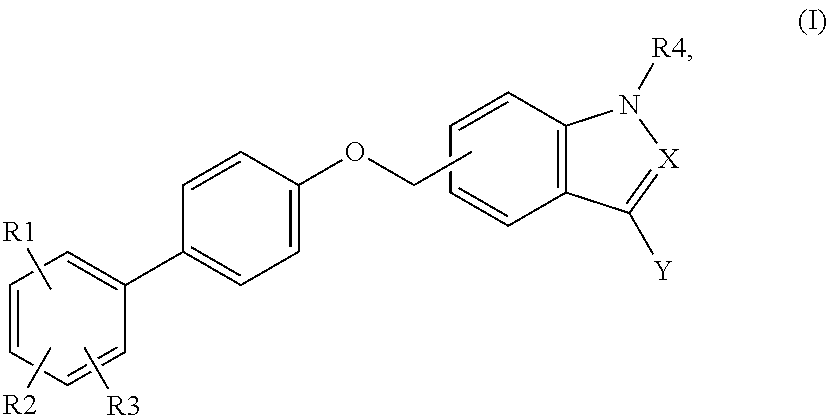
Indole and indazole analogs as glycogen synthase activators
a technology of glycogen synthase and indole, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of weight gain, loss of effectiveness, and inability to control disease,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
4-(4′,5′-Difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-1H-indazole
[0085]
[0086]3-Amino-2-methyl benzyl alcohol (0.82 g, 5.98 mmol), acetic anhydride (1.68 mL, 17.8 mmol), potassium acetate (1.75 g, 17.8 mol), isoamyl nitrite (1.82 mL, 13.7 mmol) and 18-crown-6 (79 mg, 0.3 mmol) in 25 mL CHCl3 were reacted as described in EP99 / 07620. The crude product was purified by flash chromatography with a gradient from 0-35% ethyl acetate in hexanes to yield acetic acid 1-acetyl-1H-indazol-4-ylmethyl ester.
[0087]Acetic acid 1-acetyl-1H-indazol-4-ylmethyl ester was treated with 6 mL 48% HBr with stirring overnight and then refluxed for 5 hrs. The reaction mixture was concentrated, diluted with CH3CN and the precipitated solid was filtered off. The residue was treated with dihydropyran (0.53 g, 6.28 mmol) in 25 mL THF and heated to reflux for 5 hrs. The reaction was cooled and distributed between CH2Cl2 and saturated NaHCO3. The organic layer was separated, washed with H2O and concentrated in vacuo....
example 2
3-[4-(4′,5′-Difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-indazol-1-yl]-propionic acid
[0090]
[0091]4-(4′,5′-Difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-1H-indazole (0.09 g, 0.245 mmol), ethyl bromopropionate (0.045 g, 0.245 mmol), cesium carbonate (81 mg, 0.294 mmol) in 6 mL DMF was stirred at RT overnight and then heated to 90° C. for 35 min. The reaction was cooled and distributed between EtOAc and H2O. The organic layer was separated and concentrated in vacuo. The crude product was purified by flash chromatography with a gradient from 0-30% ethyl acetate in hexanes to yield 3-[4-(4′,5′-difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-indazol-1-yl]-propionic acid ethyl ester as a white solid (98 mg, 85.7%).
[0092]3-[4-(4′,5′-Difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-indazol-1-yl]-propionic acid ethyl ester (0.095 g, 0.204 mmol) and lithium hydroxide hydrate (10 mg, 0.245 mmol) in 5 mL THF / 1 mL H2O was stirred at RT for 3 hrs. The reaction was distributed between EtOAc and H2O. The water ...
example 3
6-(4′,5′-Difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-1H-indazole
[0093]
[0094]6-(4′,5′-Difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-1H-indazole was prepared from 3-amino-4 methyl benzyl alcohol (1 g, 0.729 mmol), as described above for 4-(4′,5′-difluoro-2′-methoxy-biphenyl-4-yloxymethyl)-1H-indazole, to yield 510 mg of product. LC-MS (ES) calculated for C21H16F2N2O2, 366.37. Found m / z 367 [M+H]+.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com