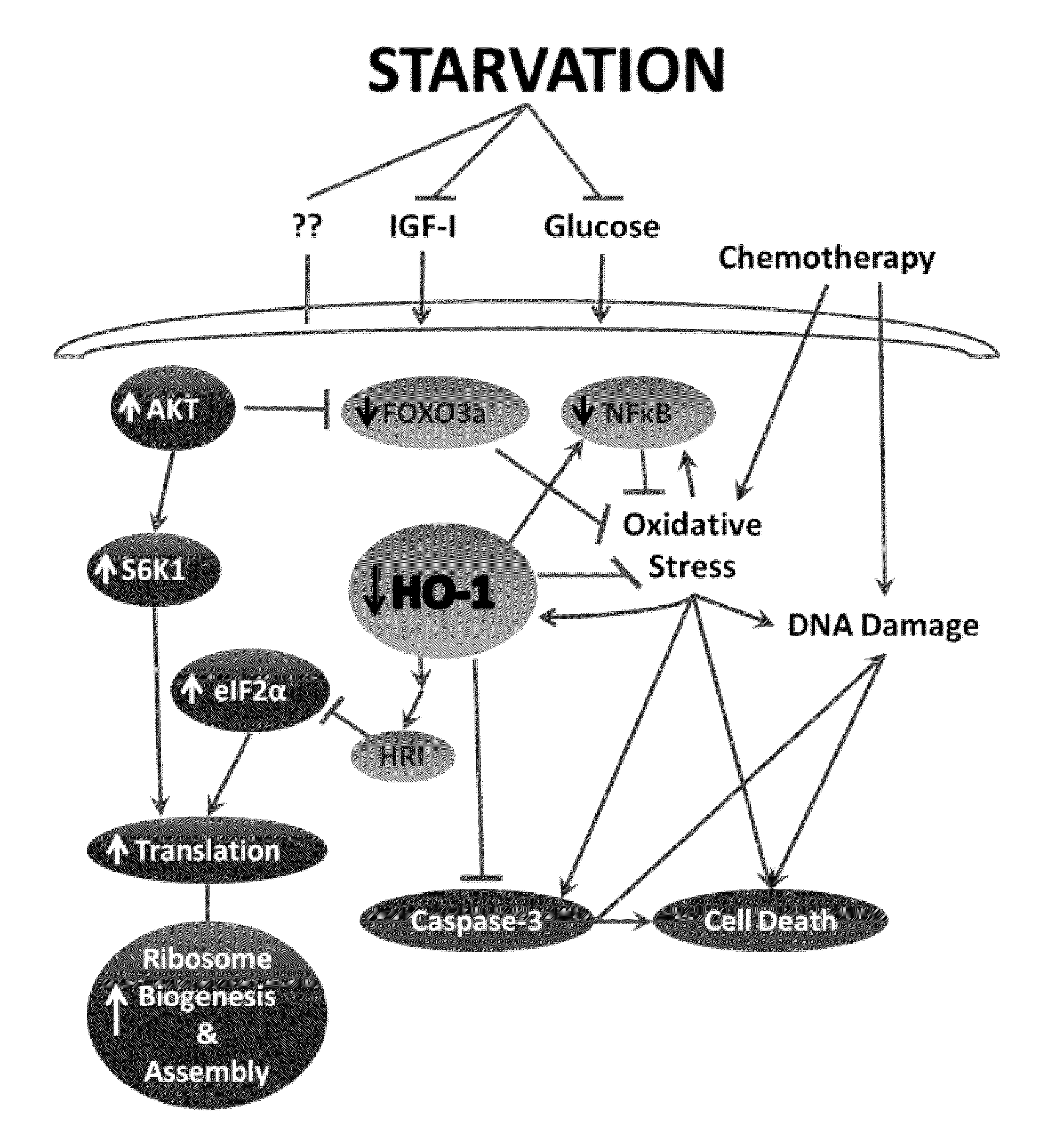
Methods and Nutritional Formulations to Increase the Efficacy and Reduce the Side Effects of Cancer Treatment
a cancer treatment and side effect technology, applied in the field of diet and cancer treatment, can solve the problems of limiting the intensity, frequency and efficacy of chemotherapy, and cannot be combined with chemotherapy nor applied alone, and achieve the effects of reducing weight, reducing cancer growth or a symptom of cancer, and reducing side effects of chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
[0105]Ten cases are described in which patients diagnosed with a variety of malignancies have voluntarily fasted prior to (48-140 hours) and / or following (24-56 hours) chemotherapy. None of these 10 patients, who received an average of 4 cycles of chemotherapy in combination with fasting, reported significant side effects caused by the fasting itself other than hunger. Toxicity was graded based on the Common Toxicity Criteria (CTC) of the National Cancer Institute (NCI) and self-reported side-effects from five patients indicate that fasting may protect against fatigue, weakness, and gastrointestinal side effects. In those patients whose cancer progression could be monitored, fasting did not prevent the chemotherapy-dependent reduction of tumor mass or tumor markers. Although controlled clinical trials are required to determine the role of fasting in the enhancement of clinical outcomes and the patient's quality of life, the 10 cases presented here suggest that fasting in combination...
case 1
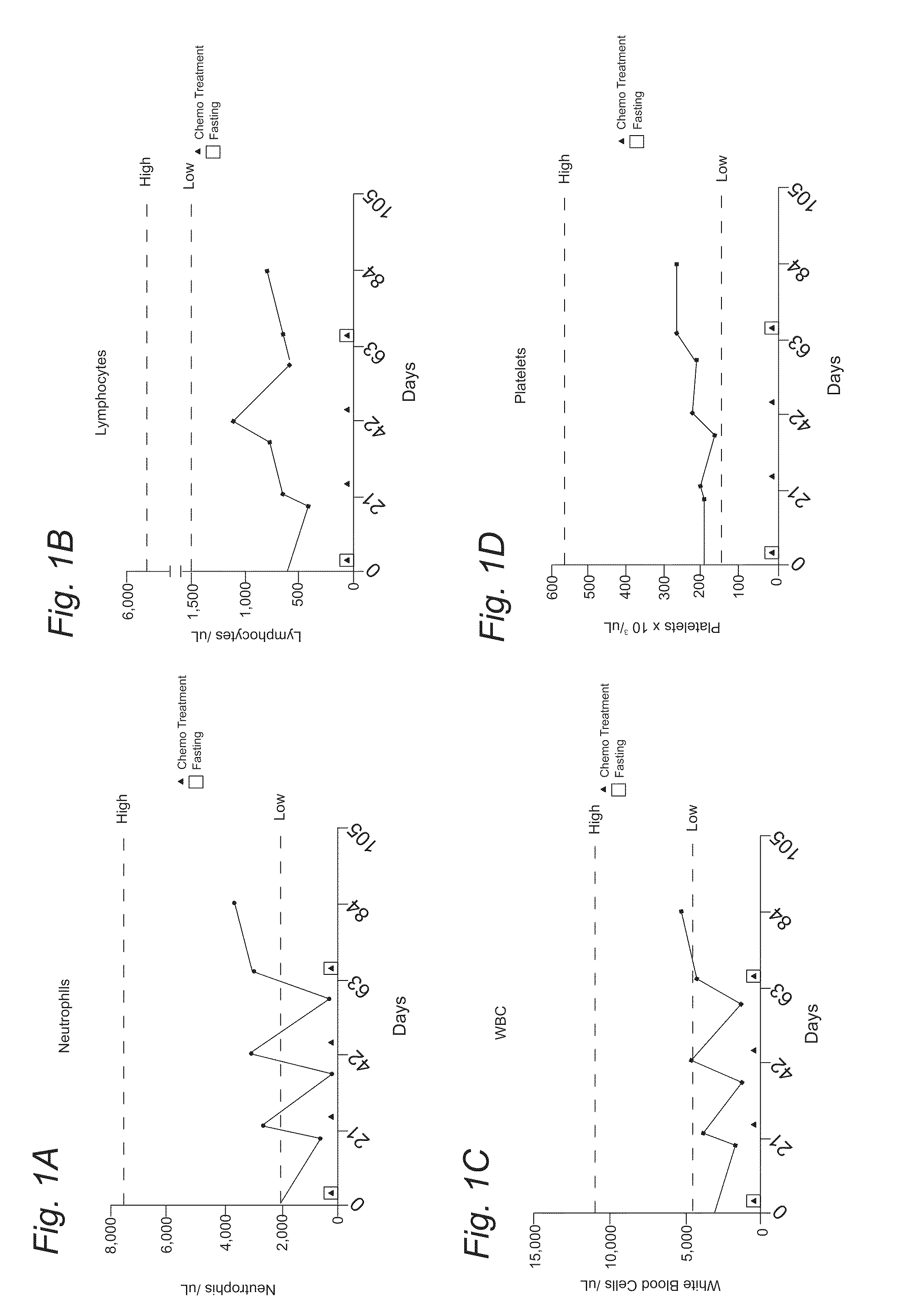
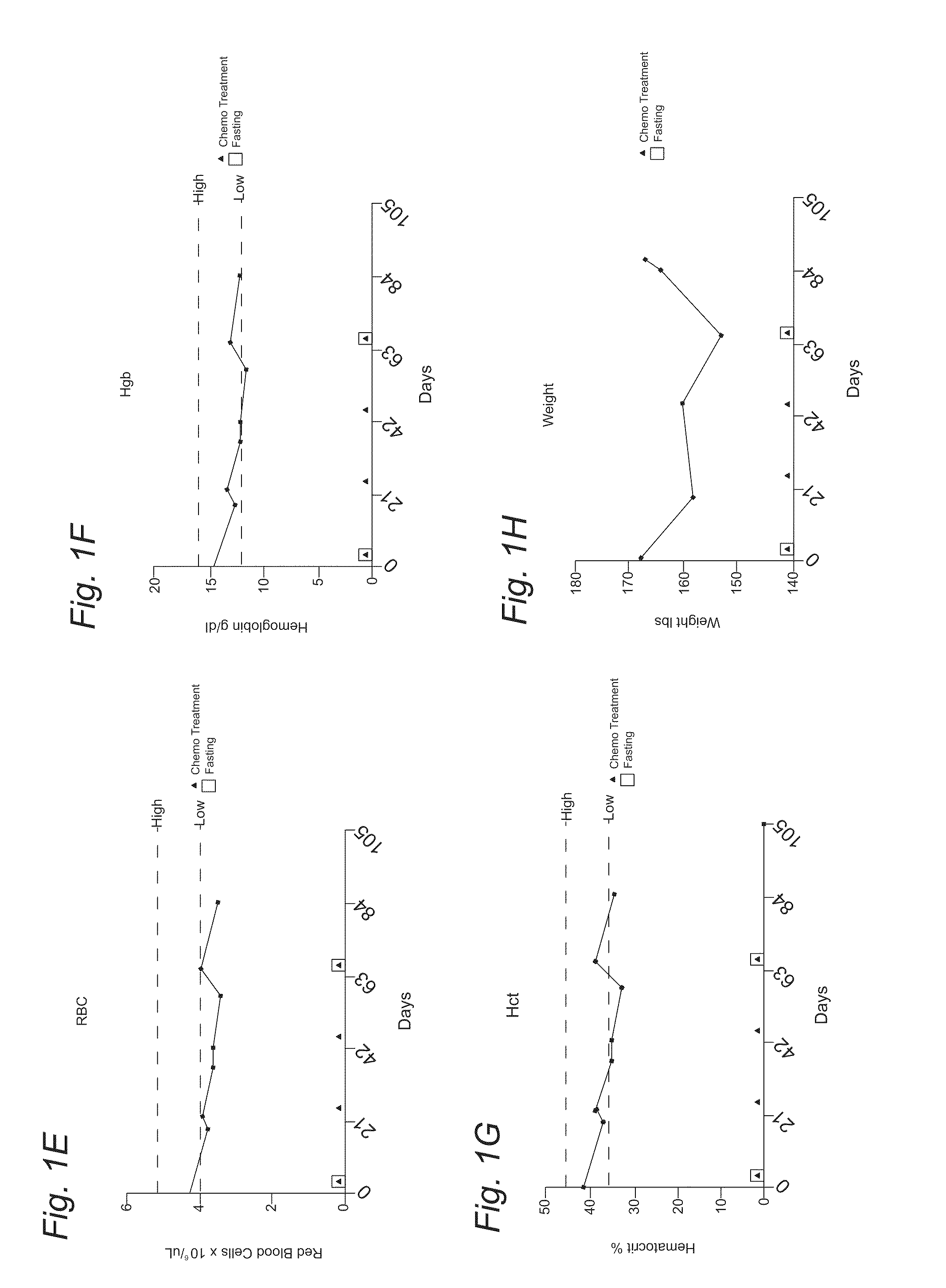
[0107]A 51-year-old Caucasian woman diagnosed with stage HA breast cancer to whom adjuvant chemotherapy with docetaxel (DTX) and cyclophosphamide (CP) was recommended. She fasted prior to her first chemotherapy administration. The fasting regimen consisted of a complete caloric deprivation for 120 hours prior and 60 hours after chemotherapy (180 hours total), during which she consumed only water and vitamins. The patient completed this prolonged fasting without major inconvenience and lost 7 pounds which were recovered by the end of the treatment (Figure IH). After the fasting-chemo cycle, the patient experienced mild fatigue, dry mouth and hiccups (FIG. 2); nevertheless she was able to carry her daily activities (working up to 12 hours a day). By contrast, in the subsequent chemo-treatment cycles (second and third), she received chemotherapy without fasting and complained of moderate to severe fatigue, weakness, nausea, abdominal cramps and diarrhea (FIG. 2). This time the side eff...
case 2
[0108]A 68-year-old Caucasian male who was diagnosed in February 2008 with esophageal adenocarcinoma. By the time of diagnosis, metastasis to the left adrenal gland was found on a CT-PET scan, consistent with stage IV disease. The initial treatment was 5-fluorouracil (5-FU) combined with cisplatin (CDDP). Concurrently with this chemotherapy, he also received localized radiation for the first two cycles. Throughout this period the patient experienced multiple side effects including severe weakness, remarkable fatigue, intense vomiting and significant peripheral neuropathy (FIG. 3). Additionally, the patient complained of intense dysphagia secondary to severe mucositis, most likely caused by the radiation treatment. During the third cycle, 5-FU administration had to be withdrawn due to severe nausea and refractory vomiting (FIG. 3). In spite of the aggressive approach with chemotherapy and radiation, his disease progressed. Development of new metastases to the right adrenal gland, low...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com