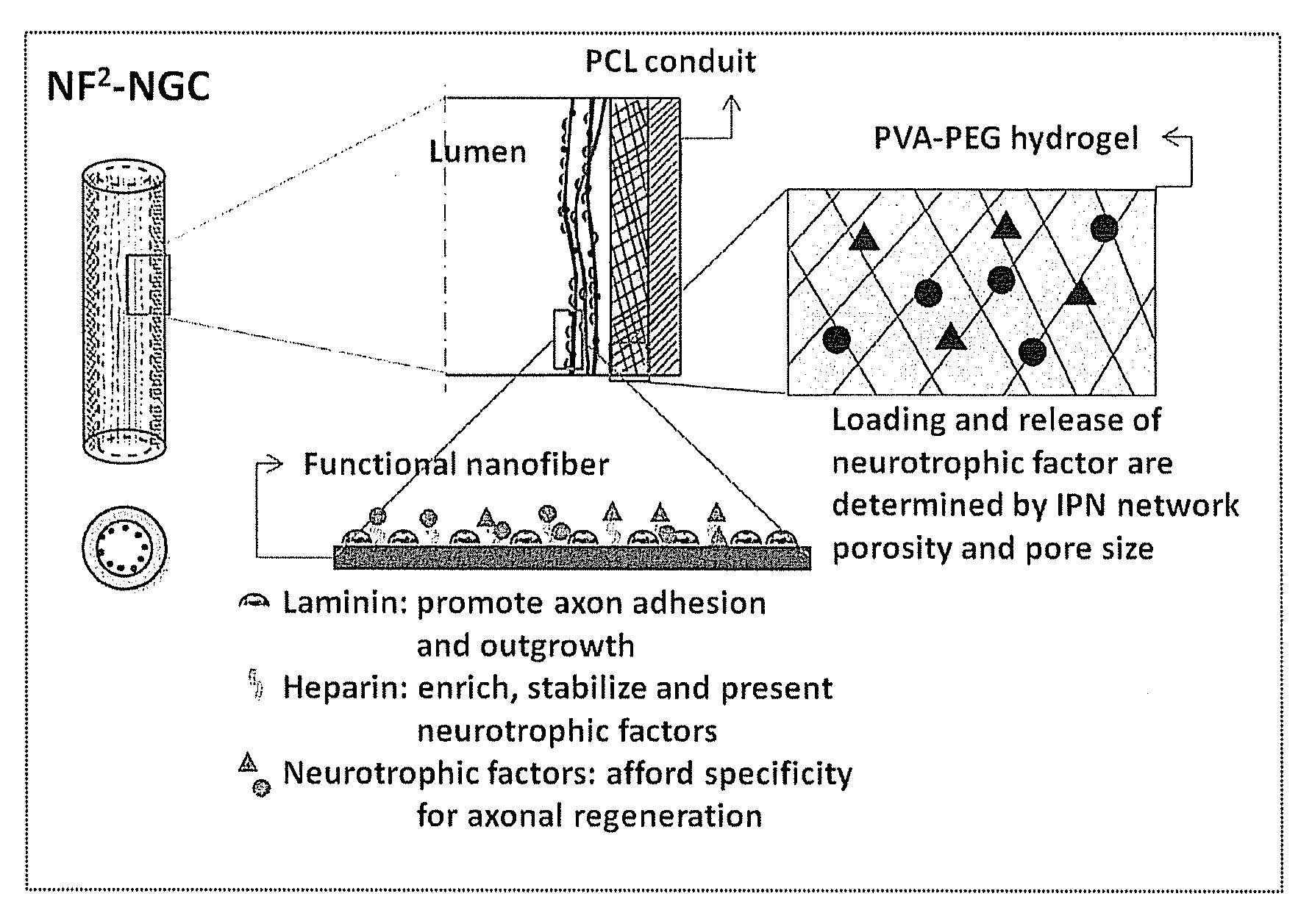
Hydrogel-grafted degradable nerve guides
a degradable, nerve guide technology, applied in the direction of prosthesis, paper/cardboard containers, synthetic resin layered products, etc., can solve the problems of physical discontinuity of a peripheral nerve, leakage of growth factors from the nerve guide, and two uncoupled nerve ends, etc., to achieve sustained release and low protein burst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Nerve Guide
[0079]1) Cast a 10 cm2 film of 50:50 PCL / PCLEEP on top of a glass slide mold from a 12% wt solution in dichloromethane.
[0080]2) Dry the film under vacuum over night to remove residual solvent.
[0081]3) Place slide on a hot plate and heat the film to 100° C.
[0082]4) Add 1 ml of 1% PVA in water on top of the film.
[0083]5) Quench the film at −20° C. for 30 minutes.
[0084]6) Add 500 μL of 5% PVA containing 5 μg GDNF on top of the film.
[0085]7) Freeze the composite at −20° C. for 12 hours.
[0086]8) Cool at 25° C. for 30 minutes.
[0087]9) Freeze the composite at −20° C. for 2 hours.
[0088]10) Cool at 25° C. for 30 minutes.
[0089]11) Repeat steps 9 and 10 for a total of 12 cycles.
[0090]12) Freeze-dry the construct.
[0091]13) Place electrospun PCL nanofibers modified with laminin on top of the hydrogel layer.
[0092]14) Roll the construct into a tube with inner diameter of 3 mm and the hydrogel layer on the luminal side.
[0093]15) Seal the membrane with a trace quantity of...
example 2
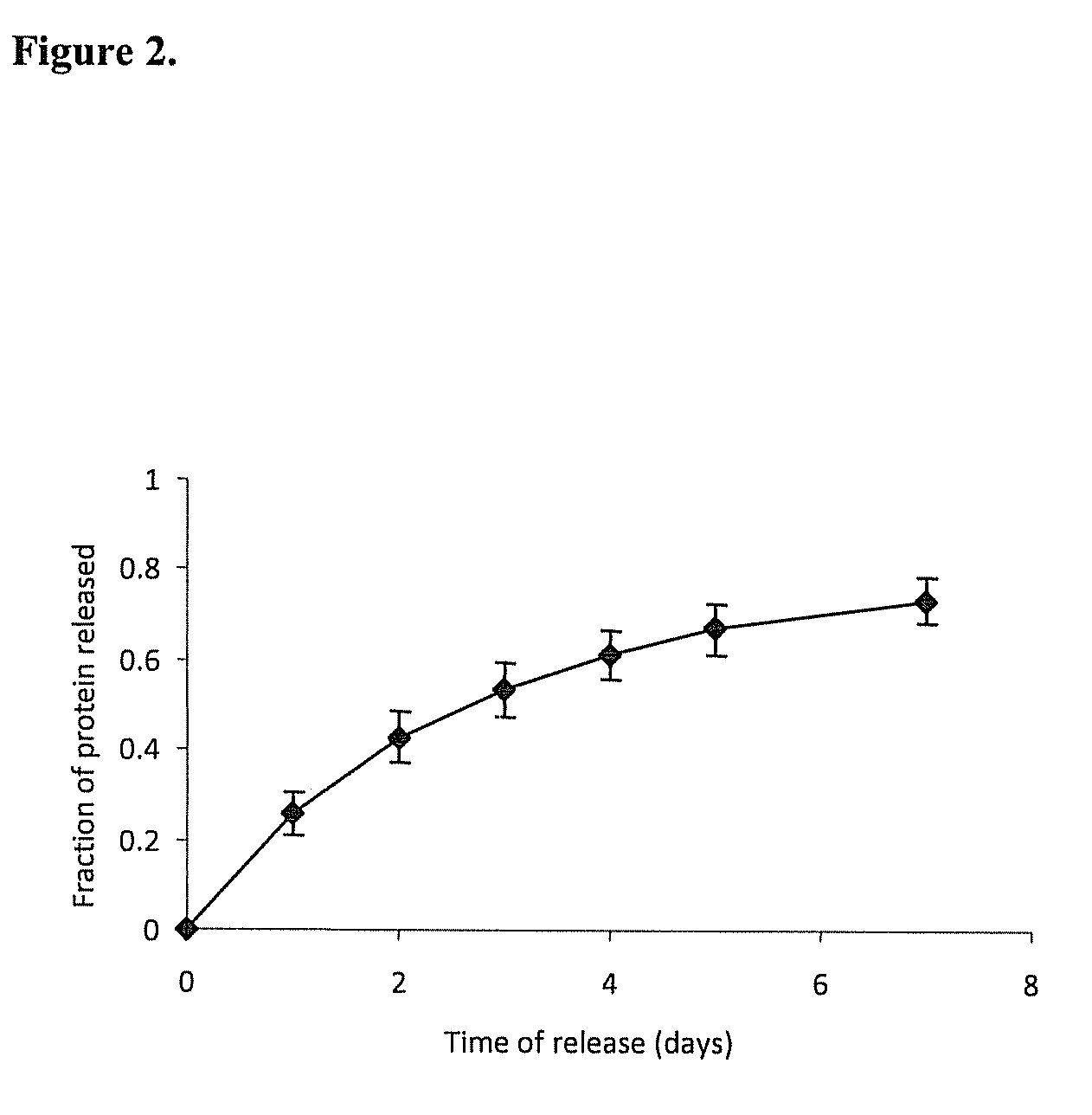
Release Profile of Model Proteins from the Nerve Guide Construct
[0095]A nerve guide is prepared as described in Example 1 with bovine serum albumin
[0096](BSA) as a model protein factor loaded in a 5% PVA-83k-99 hydrogel construct into buffered saline at 37° C., gel was frozen at −2° C. for 72 hours, followed by 4 conventional freeze-thaw cycles. The release profile of BSA is shown in FIG. 2.
example 3
Process of Preparing a Double-Layered Hydrogel Nerve Guide
[0097]1) Repeat steps 1-8 as described in Example 1 with Gel A loaded onto the PCL membrane.
[0098]2) Independently prepare a 0.5 mm thick PVA hydrogel (Gel B) by replicating steps 6-11 as described in Example 1, with the sole modification of omitting the growth factor loading.
[0099]3) Add 50 μL of 5% PVA solution on top of Gel A, then lay Gel B on top of the solution.
[0100]4) Repeat steps 9 and 10 as described in Example 1 for a total of 4 cycles.
[0101]5) Continue with steps 12-16 as described in Example 1.
[0102]Other modifications can be used to modify the rate of release of proteins from the hydrogel. The addition of a protein-free top layer provides an additional diffusion barrier to incorporate time-delayed protein release. Blending PVA with other water-soluble small molecular weight polymers changes the hydrogel crystallinity. Changing the number of freeze-thaw cycles, or simply incubation for prolonged periods at sub-ze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com