Use of proline specific endoproteases to hydrolyse peptides and proteins
a technology of proline and endoprotease, which is applied in the direction of peptide/protein ingredients, immunological disorders, metabolism disorders, etc., and can solve the problems of unsatisfactory effects in specific groups of individuals, the presence of such molecules in protein hydrolysates, and the use of proline specific endoproteases,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
The Enzyme as Obtained from A. Niger Represents a New Class of Proline Specific Enzymes
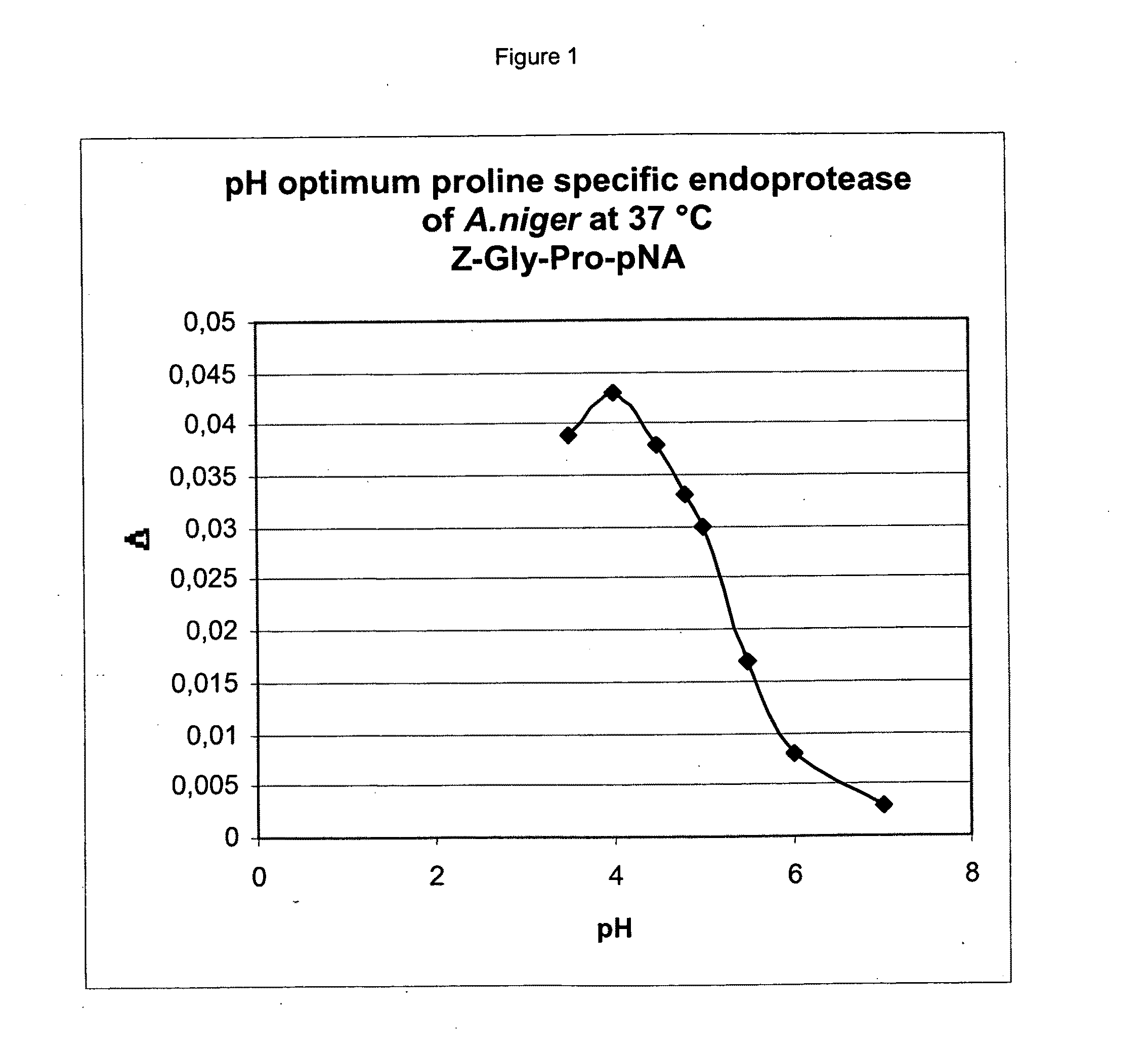
[0098]From the entire coding sequence of the A. niger derived proline specific endoprotease as provided in WO 02 / 45524 a protein sequence of 526 amino acids can be determined. The novelty of the enzyme was confirmed by BLAST searches of databases such as SwissProt, PIR and trEMBL. To our surprise, no clear homology could be detected between the A. niger enzyme and the known prolyl oligopeptidases. Closer inspection of the amino acid sequence, however, revealed low but significant homology to Pro-X carboxypeptidases (EC3.4.16.2), dipeptidyl aminopeptidases I (EC3.4.14.2), and thymus specific serine protease. All of these enzymes have been assigned to family S28 of serine peptidases. Also the GxSYxG configuration around the active site serine is conserved between these enzymes and the A. niger derived endoprotease. Additionally, members of family S28 have an acidic pH optimum, have specificity for c...
example 3
The A. Niger Derived Proline Specific Endoprotease can Hydrolyse Large Proteins as Well as Small Peptides and is Thus a True Endoprotease
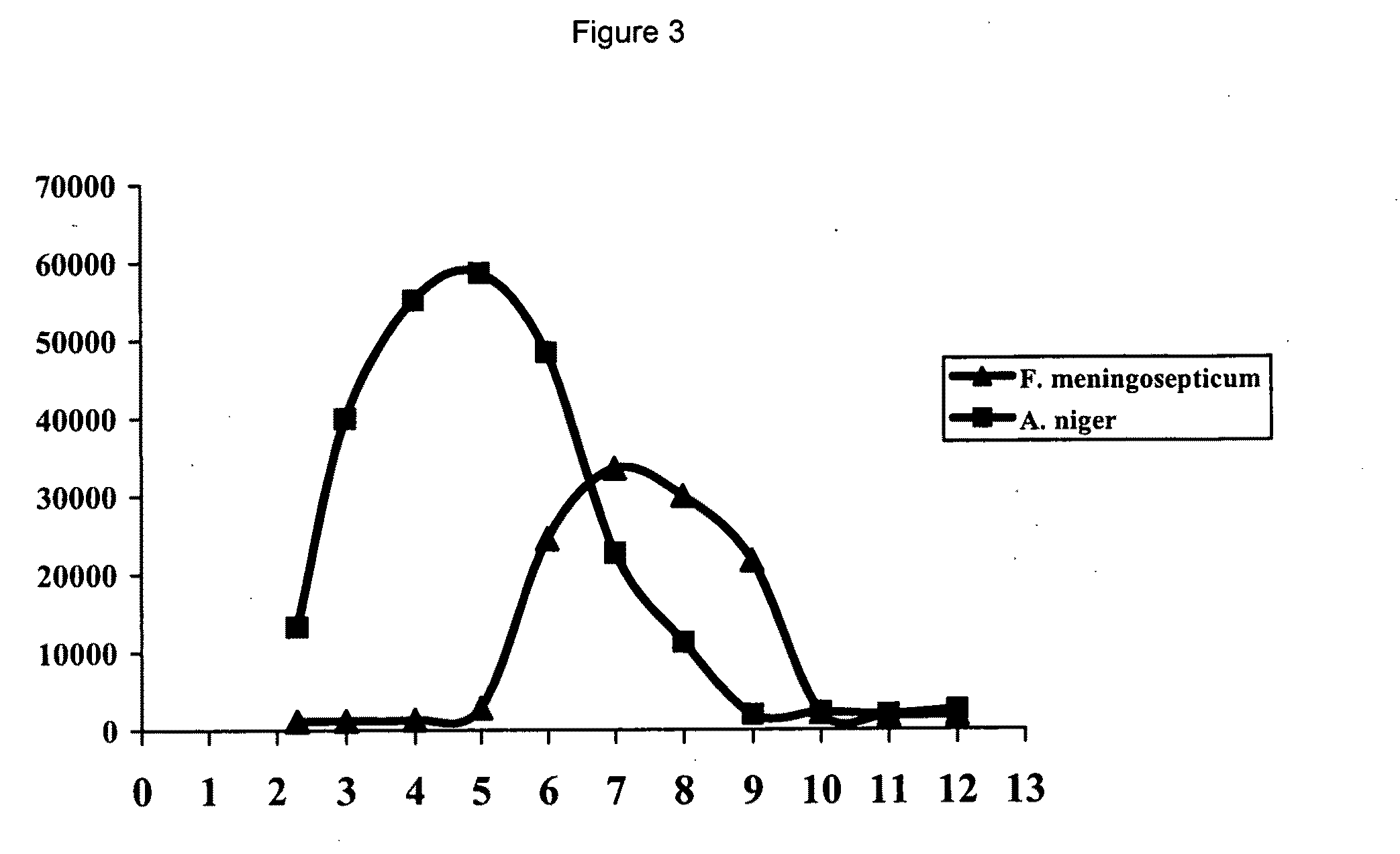
[0100]Owing to a specific structural feature, prolyl oligopeptidases belonging to the S9 family cannot digest peptides larger than 30 amino acids. This limitation is an obvious disadvantage for an enzyme, which is meant to hydrolyse as quickly and as efficiently as possible all potential proline rich toxic proline rich peptides. To see if the A. niger derived proline specific endoprotease exhibits the same limitations with respect to the size of the substrate molecule, we have incubated the chromatographically purified prolyl endopeptidase from A. niger with a small synthetic peptide and with the large ovalbumine molecule and have analysed the hydrolysis products formed by SDS-PAGE. The synthetic peptide used was a 27-mer of the sequence NH2-FRASDNDRVIDPGKVETLTIRRLHIPR-COOH and was a gift of the Pepscan company (Lelystad, The Netherlands). As shown...
example 4
Beta-Casomorphins in Hydrolysates Formed After Incubation with Alcalase and a Combination of Alcalase Plus Proline Specific Endoprotease from A. Niger
[0108]In analogy with the formation of protease-resistant beta-casomorphins during gastro-intestinal proteolysis, we wondered whether during the industrial production of milk protein hydrolysates a similar accumulation of BCM-7 related peptide fragments would occur. To that end we incubated A2 beta-casein isolated from bovine milk with the industrially frequently used subtilisin Alcalase and with Alcalase plus the proline specific endoprotease from A. niger. Using LC / MS / MS analysis the peptides thus formed were analysed.
[0109]Bovine milk contains almost 10 grams of beta-caseine per kg of milk representing 28% of all protein present. To facilitate the analysis of BCM-7 related amino acid. sequences, in this experiment we used a concentrated preparation (from Sigma) containing a minimum of 90% (A2) beta-casein. The latter product was di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com