Method for development of a peptide building block useful for de novo protein design
a technology peptides, applied in the field of peptide building blocks useful for de novo protein design, can solve the problems of significant unresolved questions regarding the practical limitations of symmetric protein design, and achieve the effect of increasing the overall thermostability and the overall thermostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Support for the Evolution of Symmetric Protein Architecture from a Simple Peptide Motif
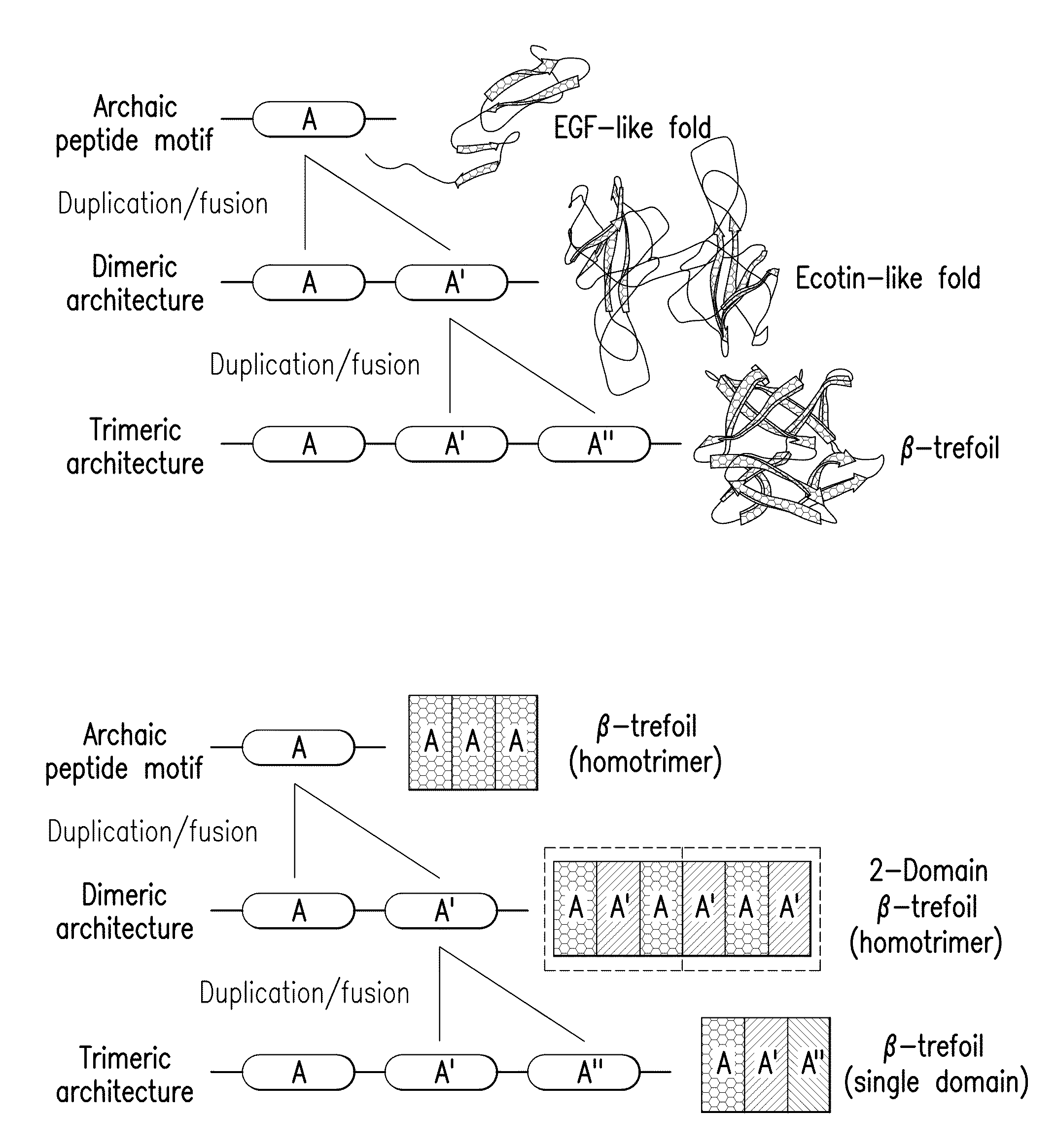
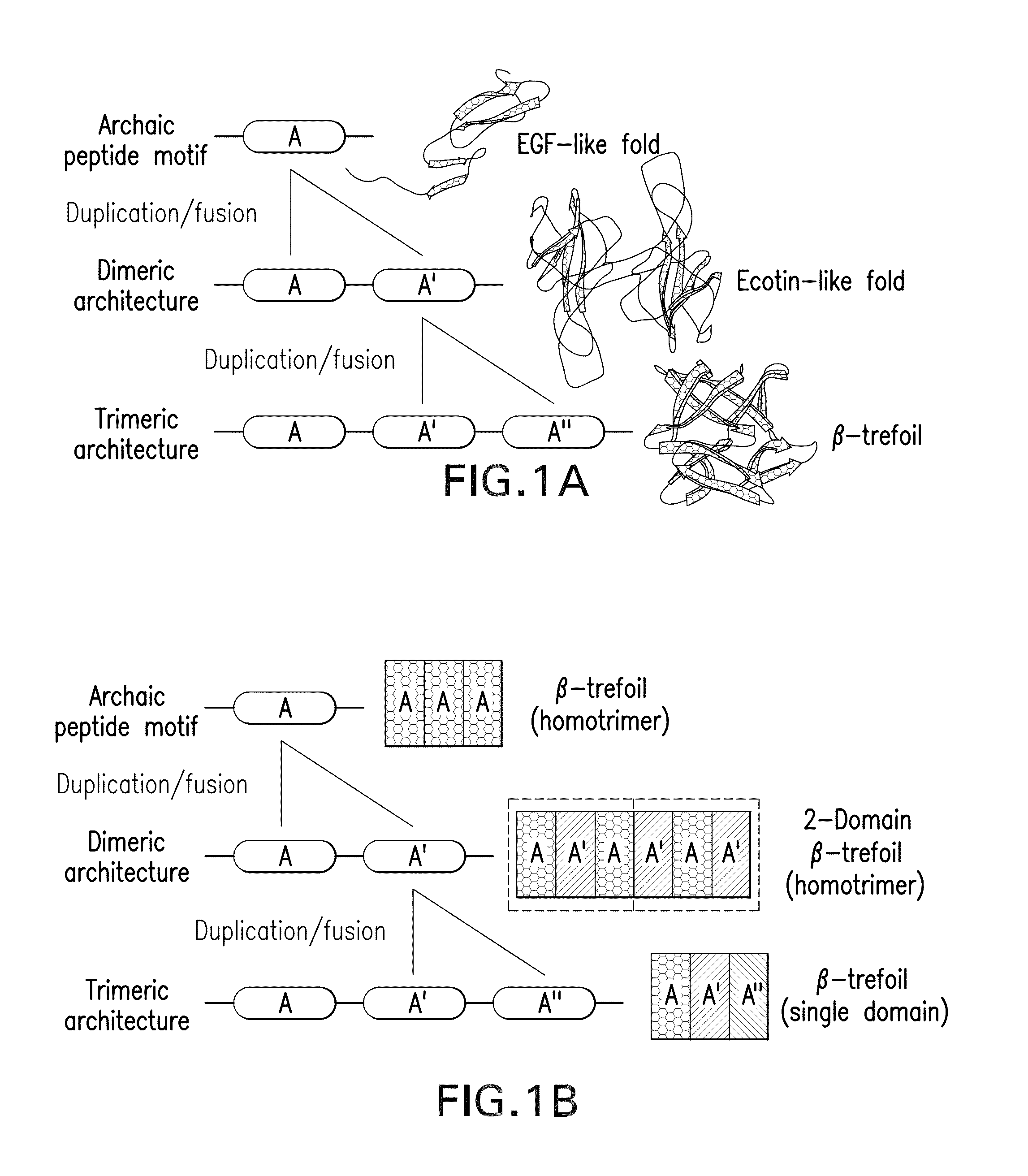
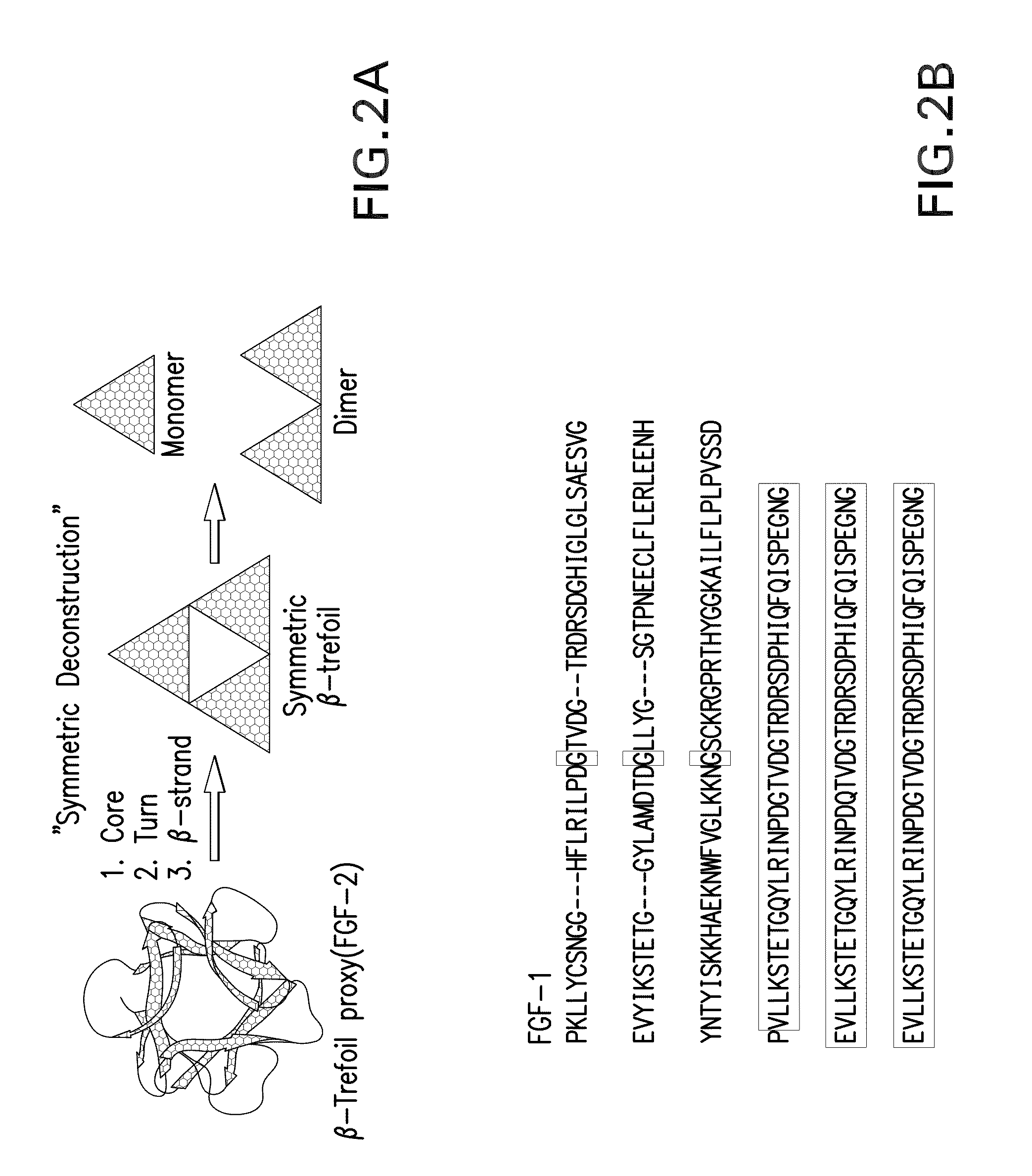
[0066]The majority of protein architectures exhibit elements of structural symmetry, and “gene duplication and fusion” is the evolutionary mechanism generally hypothesized to be responsible for their emergence from simple peptide motifs. Despite the central importance of the gene duplication and fusion hypothesis, experimental support for a plausible evolutionary pathway for a specific protein architecture has yet to be effectively demonstrated. To address this question, a unique “top-down symmetric deconstruction” strategy was utilized to successfully identify a simple peptide motif capable of recapitulating, via gene duplication and fusion processes, a symmetric protein architecture (the threefold symmetric β-trefoil fold). The folding properties of intermediary forms in this deconstruction agree precisely with a previously proposed “conserved architecture” model for symmetric prote...
example 2
Supporting Information for Experimental Support for the Evolution of Symmetric Protein Architecture from a Simple Peptide Motif
[0082]Mutagenesis and Protein Purification. Construction of all the “SYMΔΔ” mutants utilized a synthetic gene for the 140 amino acid form of human fibroblast growth factor-1 (FGF-1) (Gimenez-Gallego et al. (1986) Biochem Biophys Res Commun 128:611-617, Linemeyer D L et al. (1990) Growth Factors 3:287-298, Ortega S et al. (1991) J Biol Chem. 266:5842-5846 and Blaber et al. (1996) Biochemistry 35:2086-2094) containing an additional amino-terminal six His tag (SEQ ID NO: 3) and following previously described procedures (Brych et al. (2001) Protein Sci 10:2587-2599). Construction of the Symfoil-1 (for symmetric β-trefoil protein 1) mutant involved complete gene synthesis utilizing unique codons at symmetry-related positions. Expression and purification of recombinant proteins followed previously published procedures (Brych et al. (2001) Protein Sci 10:2587-2599)...
example 3
A Polypeptide “Building Block” for the β-Trefoil Fold Identified by “Top-Down Symmetric Deconstruction”
[0089]Transform #1: Top-down SD of core positions. Attempts to introduce a symmetric constraint on the hydrophobic core-packing group, without also making alterations to the asymmetric FGF-1 tertiary structure, met with limited success. An alternative core-packing group involving five residue positions (SYM5 mutant, FIG. 8) was identified that was essentially equivalent to the FGF-1 thermostability and folding cooperativity (Brych, S. R. et al. (2001). Protein Sci. 10, 2587-2599 and Brych, S. R. et al. (2003) Protein Sci. 12, 2704-2718). Folding and unfolding kinetic data showed that the SYM5 mutant exhibited folding and unfolding rates similar to that of the FGF-1 protein including the characteristic biphasic folding behavior (Brych, S. R. et al. (2003) Protein Sci. 12, 2704-2718). Attempts at introducing a further symmetric constraint (Ile) at symmetry-related positions 25, 67, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com