Whey protein compositions, methods and uses
a technology of whey protein and composition, applied in the field of whey protein composition, methods and uses, can solve the problems of denaturation of whey protein, poor and pronounced heat lability of whey protein in sterilizing heat treatment, etc., and achieve good textural and sensorial properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
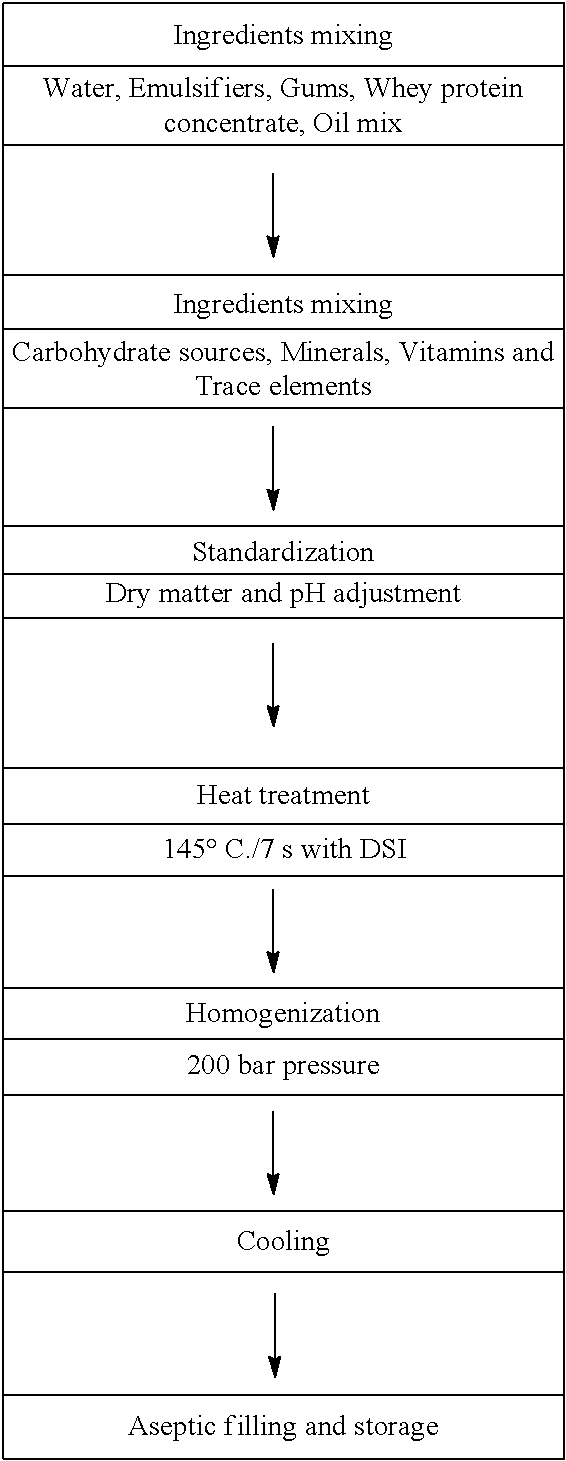
A Neutral Whey Liquid Non-Gel Composition Having High Whey Protein Content From Whey Protein Concentrate (At Least About 10 g / 100 g Or 110 g / 1)
[0131]A shelf-stable, neutral whey liquid non-gel composition having up to 100% whey protein content (up to 10 g / 100 g or 11 g / 100 ml) was prepared following the flow diagram illustrated below.
[0132]The pH range of the resulting neutral whey liquid composition is about 6.8 to about 7.2. Other properties of the neutral whey liquid composition include low viscosity, pleasant sweet taste and shelf-stability for up to 9 months.
Energy (kcal / 100 g)155Total proteins (g / 100 g)9.5% Whey in Total Proteins100Total Fat (g / 100 g)6.5Total Carbohydrates (g / 100 g)15Calcium (mg / 100 g)About 56Magnesium (mg / 100 g)About 25pH (−)7.0Viscosity at 20° C., 200 s−1 (mPa · s)About 40
[0133]To achieve the desired neutral whey liquid compositions of the present invention without any protein perceivable aggregation (gelling), several conditional parameters were applied, as...
example 2
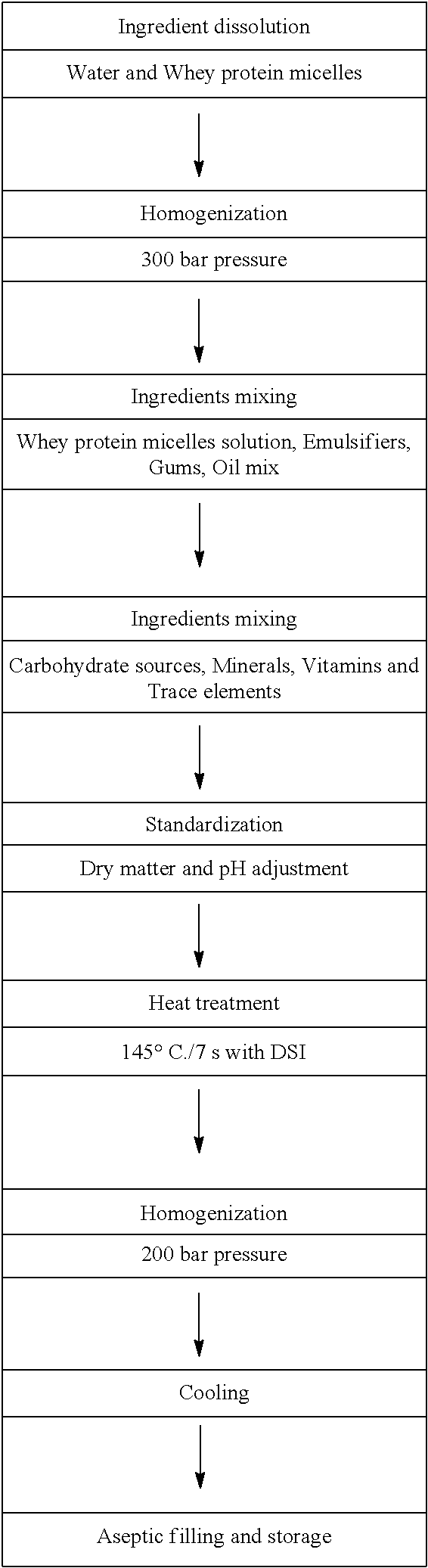
A Neutral Whey Liquid Non-Gel Composition Having High Whey Protein Content From Whey Protein Micelles (At Least About 13 g / 100 g Or 143 g / l)
[0134]A shelf-stable, neutral whey liquid non-gel composition having up to 100% whey protein content (at least about 13 g / 100 g or 143 g / 100 ml) was prepared following the flow diagram illustrated below.
[0135]The pH range of the resulting neutral whey liquid composition is about 6.8 to about 7.2. Other properties of the neutral whey liquid composition include low viscosity, pleasant sweet taste and shelf-stability.
Energy (kcal / 100 g)150Total proteins (g / 100 g)13% Whey in Total Proteins100Total Fat (g / 100 g)6Total Carbohydrates (g / 100 g)11.5pH (−)7.0Viscosity at 25° C., 200 s−1 (mPa · s) About 80
[0136]To achieve the desired neutral whey liquid composition of the present invention without any protein perceivable aggregation (gelling), conditional parameters similar to example 1 were applied, as illustrated in the flow diagram above.
example 3
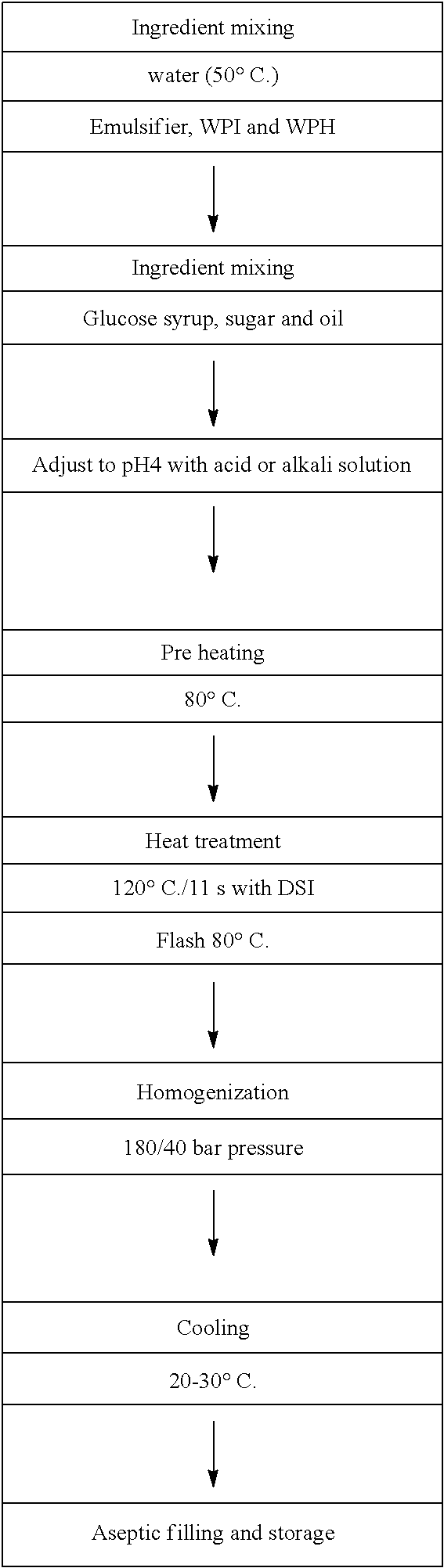
An Acid Whey Liquid Composition Having A Low Energy Content of At Least About 100 To 185 kcal / 100 g, A Total Protein Content of At Least About 13.5 g / 100 g And A Ratio of Whey Isolate:Whey Hydrolysate of At Least About 70:30
[0137]
IngredientINGREDIENTSMassIngredint NameKgGlucose Syrup9.00Whey Protein Isolate10.67Sugar9.00Phosphoric Acid0.106Vegetable Oil7.00Whey Protein Hydrolysate5.64Emulsifier0.20Total ingredients41.61Water to be added 58.39Total Finished Product100.00
[0138]The macronutrients breakdown of the acid whey liquid composition having a low energy content and a total protein content of at least about 13.5 g / 100 g are as follows:
Kcal / 100 gg / 100 g productEnergyFatProteinCarbohydrate185713.516.5
[0139]Below is a flow diagram illustrating a process for formulating the above-mentioned acid whey liquid composition having a low energy content of at least about 100 to 185 kcal / 100 g, a total protein content of at least about 13.5 g / 100 g and a ratio of whey isolate:whey hydrolysat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com