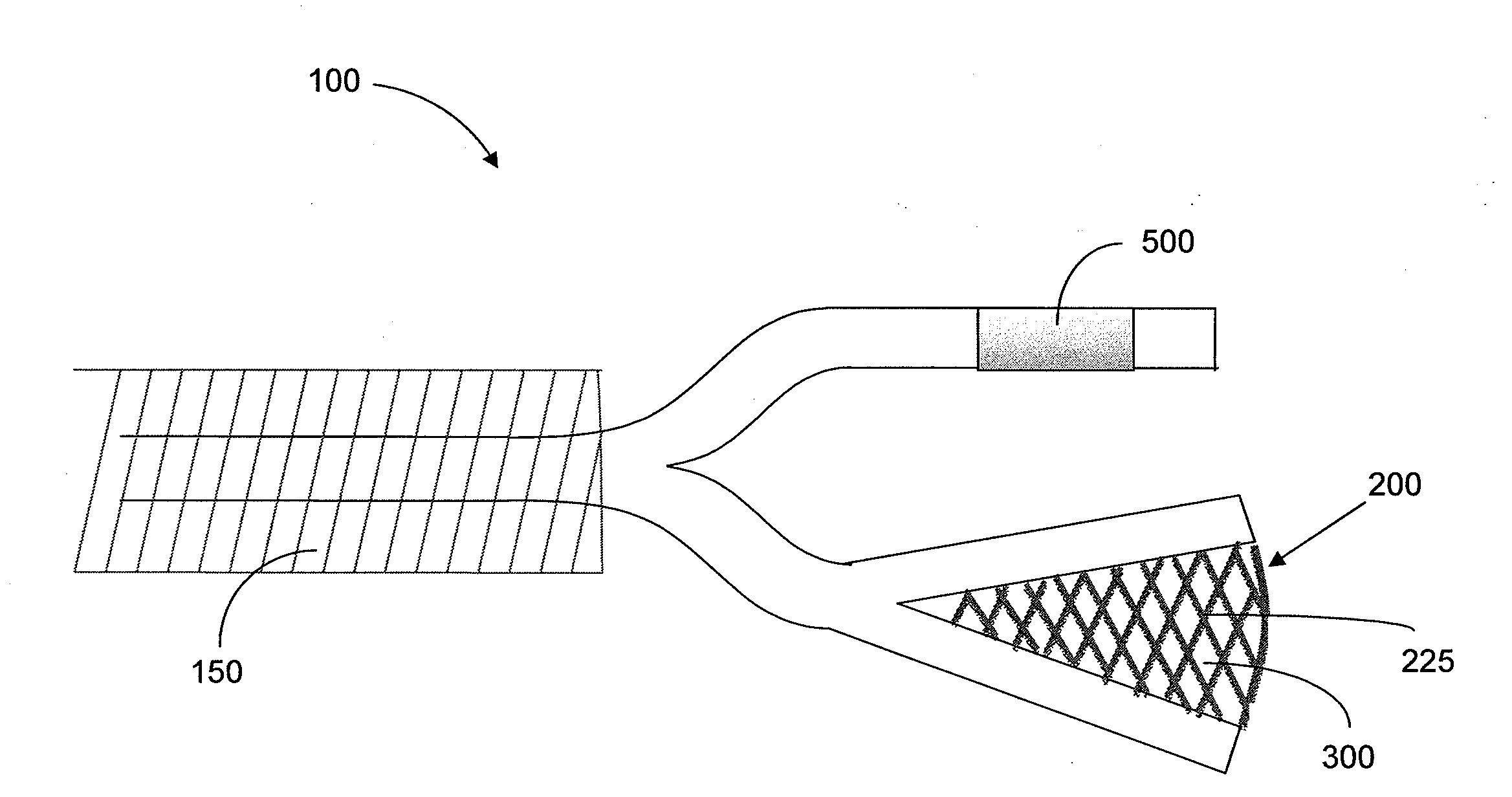
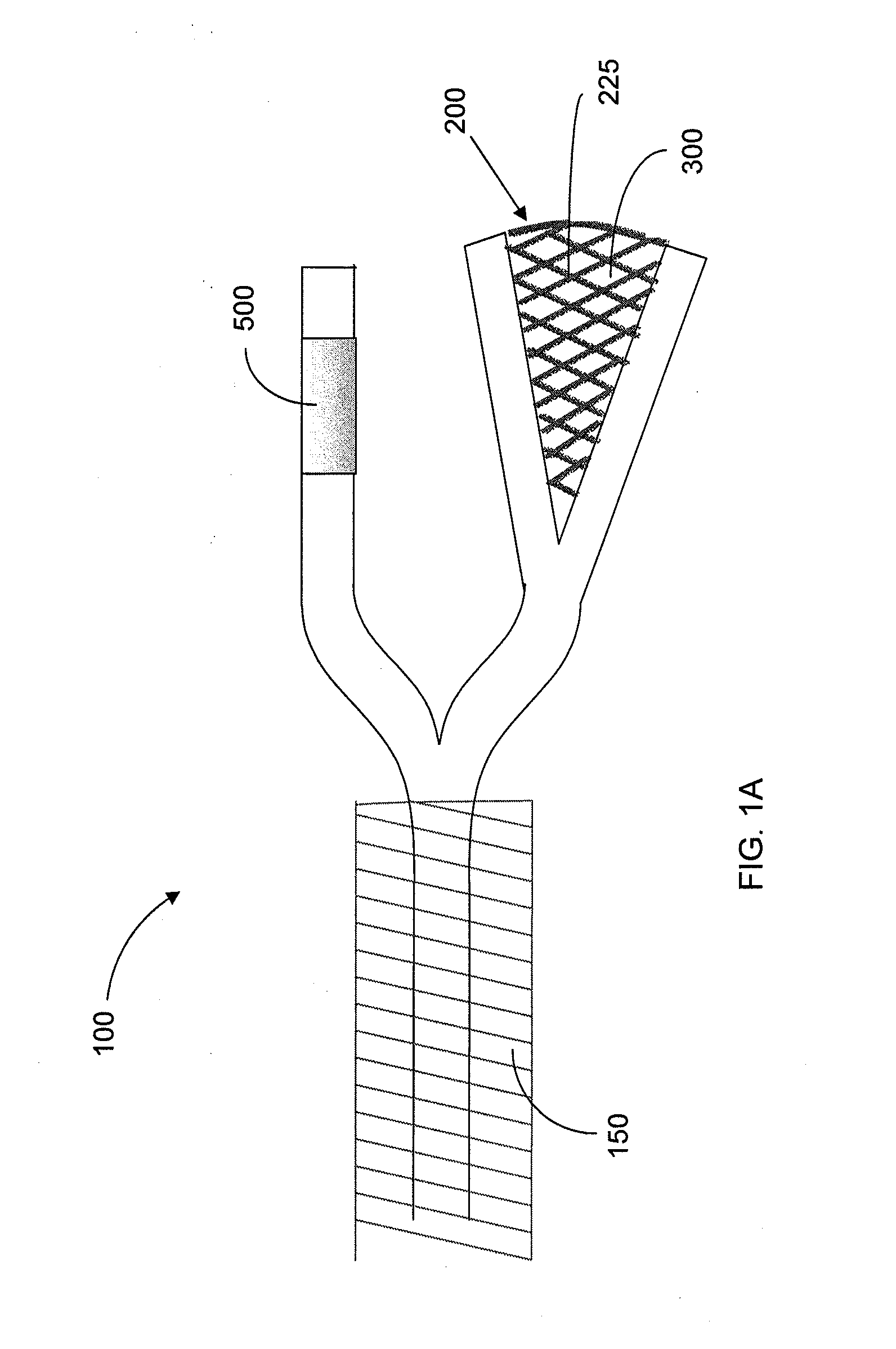
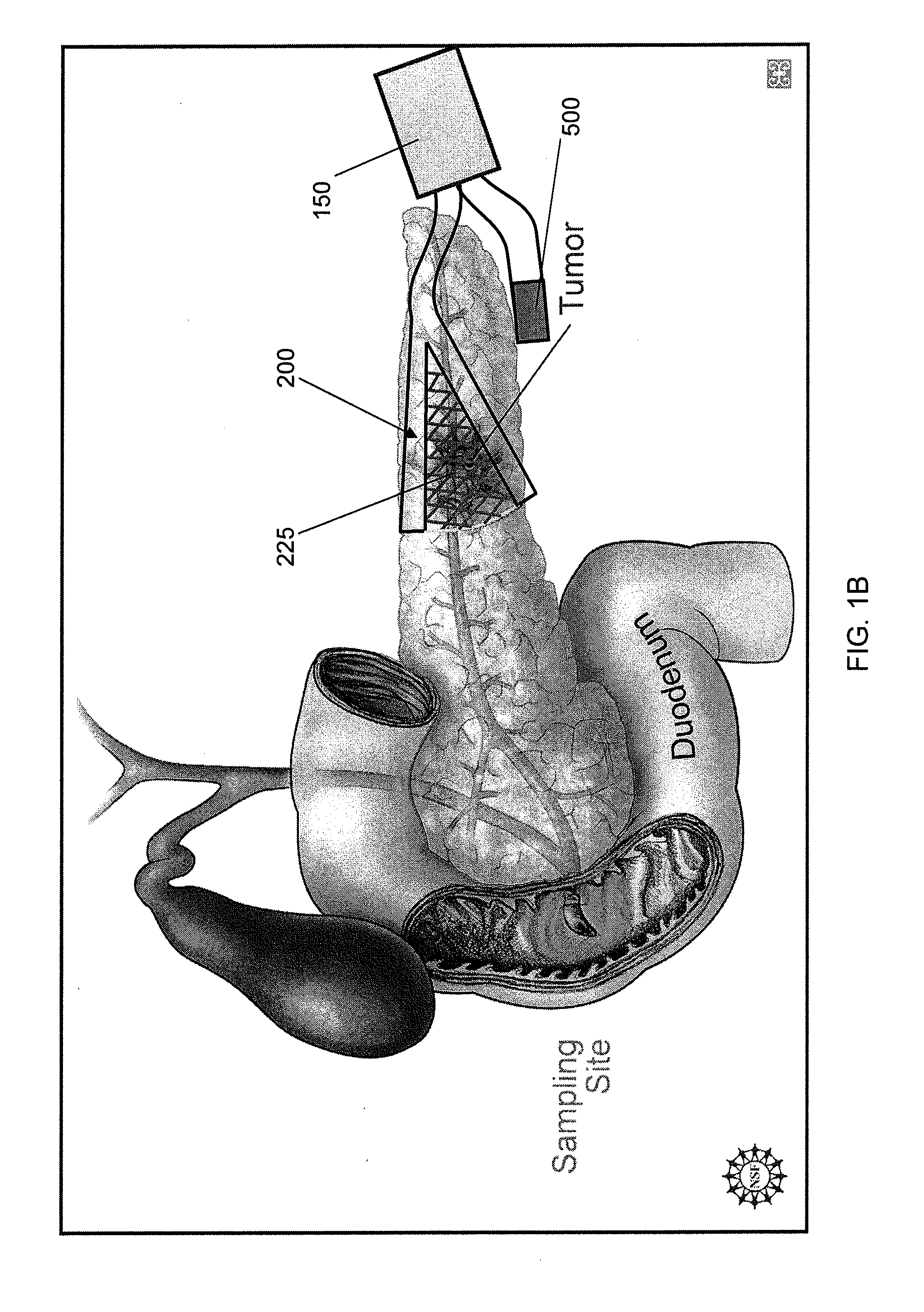
Interventional drug delivery system and associated methods
a drug delivery and drug technology, applied in the field of interventional drug delivery systems, can solve the problems of significant disadvantages of oral administration of drugs, inability to embed/secure therapeutics in tissue(s) of interest, and existing medical device technologies that enable localized placement of drugs fail to provide the opportunity to embed/secure therapeutics. the effect of high targeted and efficien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Delivery of Rhodamine 6G Dye into Agarose Phantoms
[0084]A cylindrical tube of 2% (w / v) agarose gel in deionized (D.I.) water was fabricated as a phantom with an outer diameter (o.d.)=2.5 cm and length ˜3-4 cm. A concentric reservoir for holding the dye (o.d=0.8 cm, length ˜2 cm) was cored out from the top surface along the longitudinal axis of the gel cylinder. Electrodes were fabricated out of aluminum foil (width ˜0.5 cm, length ˜15 cm, thickness ˜0.1 cm). A solution of 0.5% Rhodamine 6G in D.I. water was used to model the delivery of a small molecule drug. The dye was filled inside the cored reservoir in the agarose phantom and the source electrode (anode, in this case) was inserted into the dye reservoir. The other end of the anode was hooked to a DC power source with an alligator clip. The agarose phantom was immersed in a beaker containing 0.25×PBS solution, as shown in FIG. 17A. The cathode, a second piece of aluminum foil, was placed in the PBS beside the agarose phantom and...
example 2
Unshielded Electrode Configurations for Control Over Targeted Delivery to Specific In Vivo Locations
[0085]Unshielded electrode configurations were developed for demonstrating control over delivery to specific in vivo locations. These include electrodes fabricated out of metal wire (silver, silver chloride), metal foil (silver, platinum, aluminum) and wire mesh (aluminum), as shown in FIGS. 18A and 18B. These are representative examples, and similar designs can be fabricated with variations in size, material and additional enhancements or refinements to the basic configuration. The advantages of wire and foil electrodes shown FIG. 18A are: simplicity and ease of use, flexibility for insertion into tiny orifices and ducts, precise control over size and potential for miniaturization. Their primary limitation is their tendency for hydrolysis of the conducting fluid medium. Silver electrodes are also susceptible to oxidation, while silver chloride electrodes can get reduced to metallic. ...
example 3
Insulated Electrode Configurations for Control Over Targeted Delivery to Specific In Vivo Locations
[0086]An insulated electrode was developed to demonstrate control over targeted delivery to specific in vivo locations. By insulating a portion of the electrode surface, it is possible to control the delivery to the tissue or organ systems in a well defined fashion. For example, the flux of drug or particles will be attenuated corresponding to the insulated areas of the electrode. Aluminum foil was folded into a long rectangular shape of appropriate dimensions (length ˜10 cm, width ˜0.4 cm, thickness ˜0.1 cm). Insulating tape (width ˜1 cm) was wrapped around the foil in alternating sections. This insulated electrode was immersed in the central reservoir of an agarose phantom (2% agarose w / v in deionized water), as shown in FIG. 19A. A solution of 0.5% Rhodamine 6G in D.I. water was used to model the delivery of a small molecule drug. The dye was filled inside the cored reservoir in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com