Controlled release pharmaceutical or food formulation and process for its preparation
a technology of controlled release and food formulation, which is applied in the direction of drug compositions, amide active ingredients, plant/algae/fungi/lichens ingredients, etc., can solve the problems of loss of the effectiveness of the pharmaceutical or food form, unpredictability, and further unforeseeable alteration of the release kinetics, so as to achieve high reproducibility and improve the release profile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
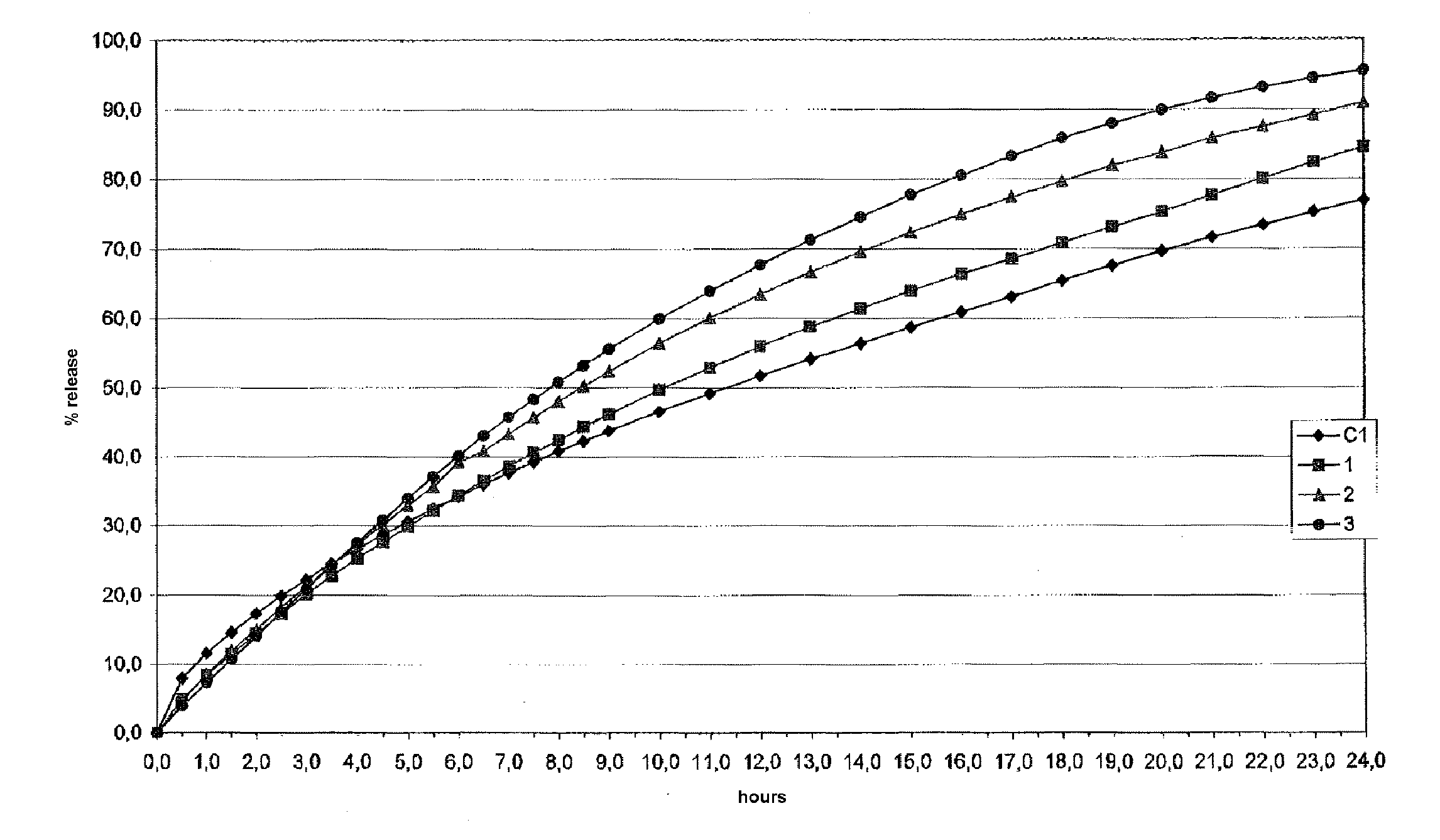
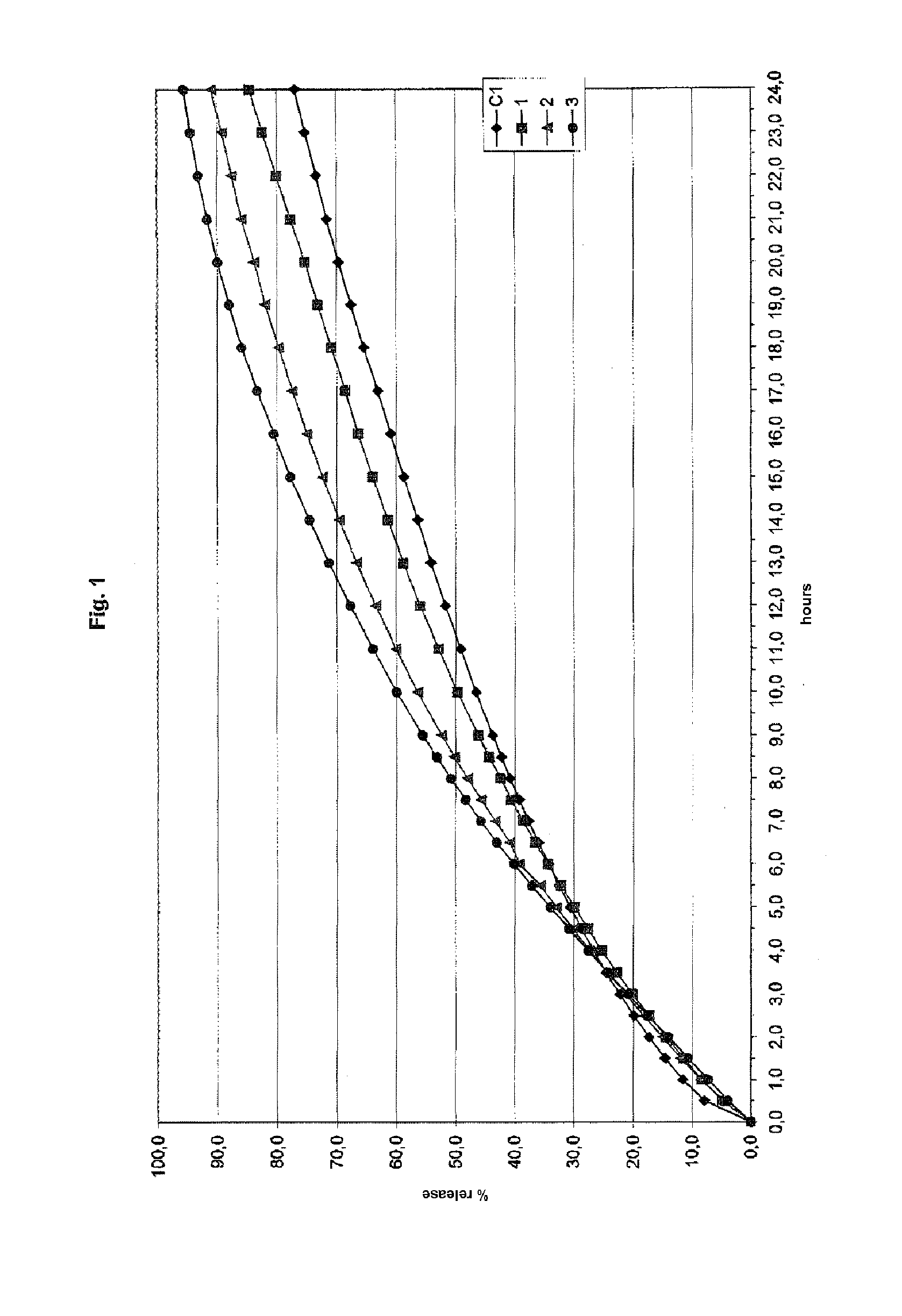
example 1
Preparation of Tablets 1-3 (Invention)
[0111]A series of tablets from 1 to 3 containing the ingredients in Table 1 were prepared using the following procedure. Excipient 2 and the glidant were mixed for approximately 2 minutes and passed through an 18 mesh sieve. Excipient 1 was first loaded into a mixer, followed by the active ingredient and finally the mixture of excipient 2 and glidant. The composition was mixed for approximately 10 minutes. Mixing was then interrupted, and the lubricant was added. After mixing for a further approximately 3 minutes the composition was discharged from the mixer and compressed in a tabletting machine.
[0112]The quantity of active ingredient, excipient 1 and excipient 2 were weighted in such a way as to give a ratio by weight between them of 3:1:1 for tablet 1, 3:1:2 for tablet 2 and 3:1:3 for tablet 3.
TABLE 1123Active ingredientParacetamol360300257Excipient 1Methocel K100M12010085.7Excipient 2Polglumyt120200257GlidantAerosil333LubricantPRUV999Polglum...
example 2
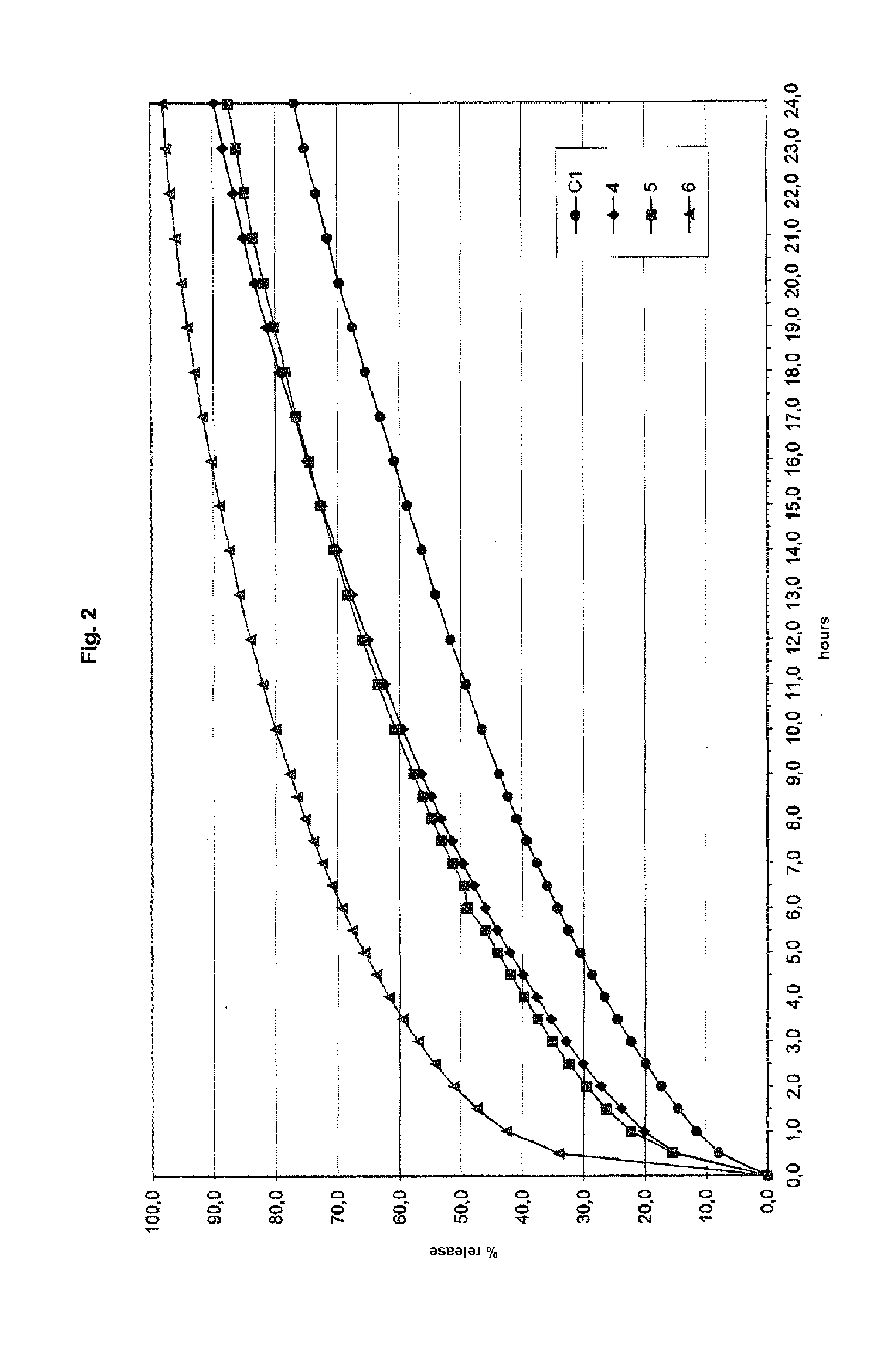
Preparation of Tablets 4-6 (Comparison)
[0116]A series of tablets from 4 to 6 containing the ingredients in Table 2 were prepared according to the same procedure as in Example 1.
TABLE 2456Active ingredientParacetamol360300257Excipient 1Methocel K100M12010085.7Excipient 2Avicel PH200120200257GlidantAerosil333LubricantPRUV999Avicel ®PH200: Microcrystalline cellulose having nominal dimensions of 180 μm, produced by FMC BioPolymer, USA
[0117]Tablets 4-6 were subjected to the same dissolution test as in Example 1 under the same conditions. The results of the dissolution test for tablets 4-6 are shown in FIG. 2.
example 3
Preparation of Tablets 7-9 (Comparison)
[0118]A series of tablets from 7 to 9 containing the ingredients in Table 3 were prepared according to the same procedure as in Example 1.
TABLE 3789Active ingredientParacetamol360300257Excipient 1Methocel K100M12010085.7Excipient 2Lactose120200257GlidantAerosil333LubricantPRUV999
[0119]Tablets 7-9 were subjected to the same dissolution test as in Example 1 under the same conditions. The results of the dissolution test for tablets 7-9 are shown in FIG. 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com