Reservoir-buffered mixers and remote valve switching for microfluidic devices
a technology of microfluidic devices and mixers, which is applied in the field of microfluidic devices, can solve the problems of high manufacturing cost, high risk of sample contamination, and loss of sample volume, and achieve the effects of low manufacturing cost, low manufacturing cost, and low cost of disposable components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
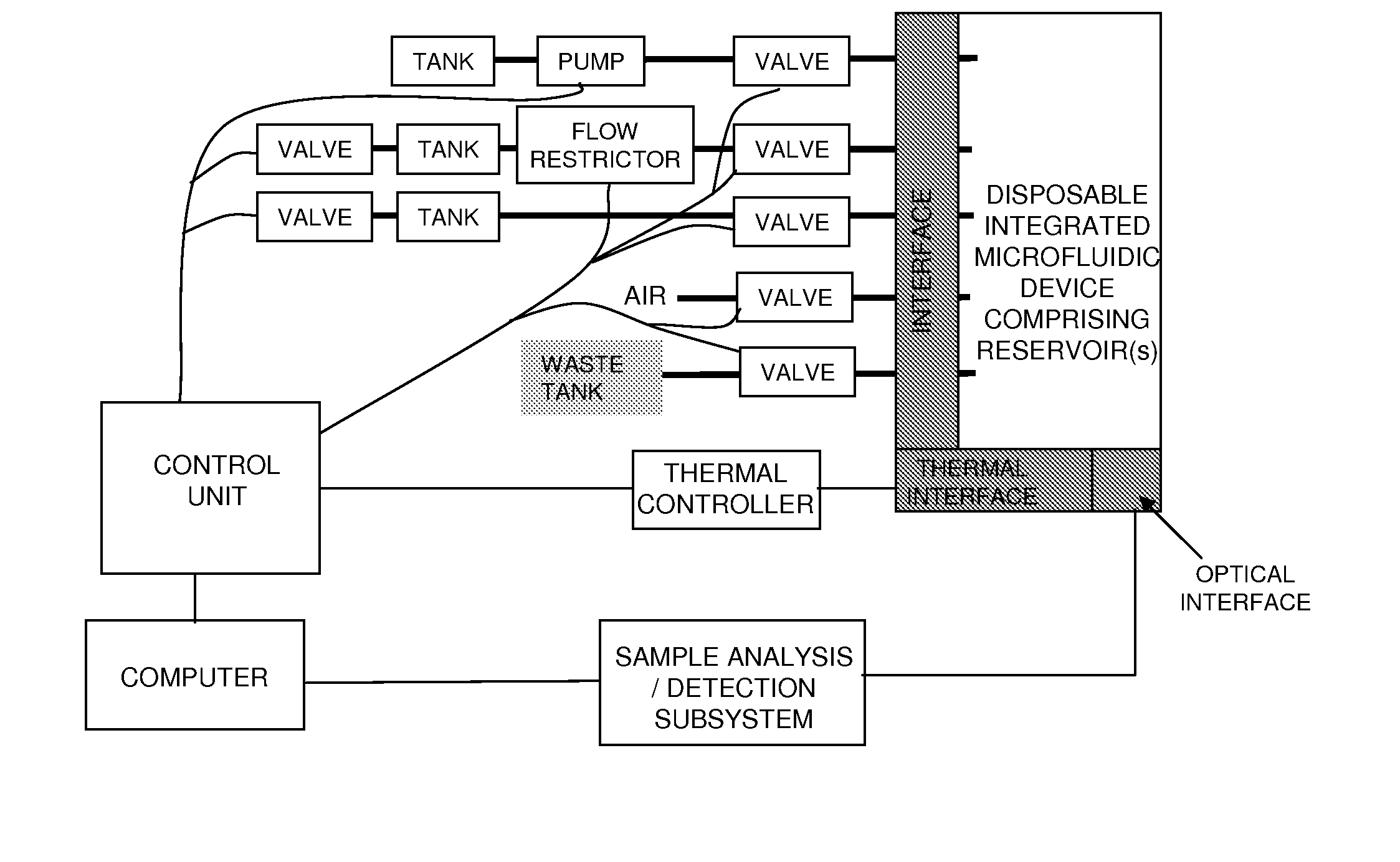
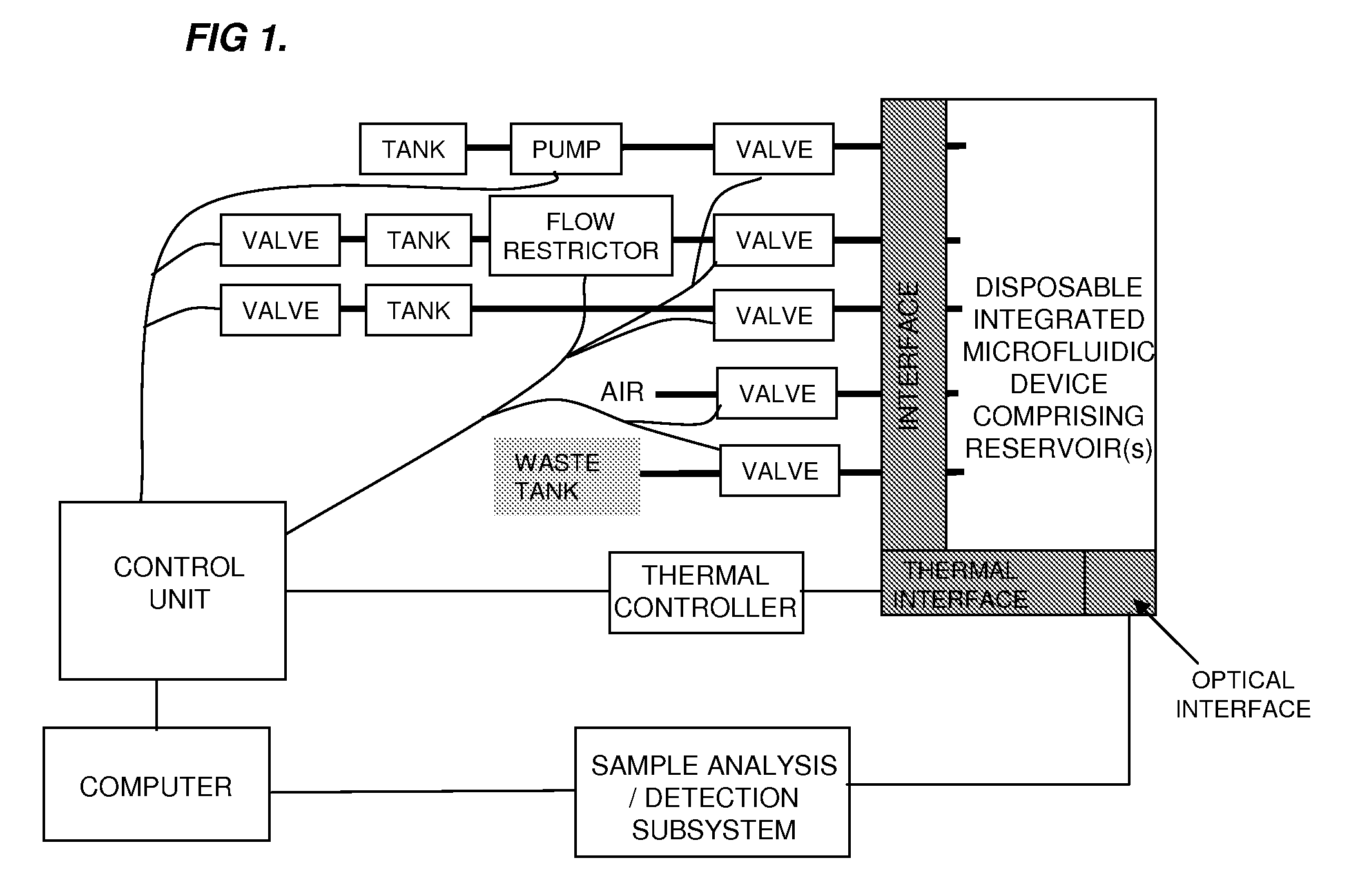
[0289]System Design. The lab-on-a-chip system was designed to accomplish the following steps:[0290]1. input 50-400 μL of liquid physiological sample containing a pathogen,[0291]2. mix the sample with a chaotropic buffer to aid in the release of nucleic acids,[0292]3. flow the mixture over the SPE column to further lyse any remaining intact pathogens and bind the nucleic acids on the SPE column,[0293]4. wash the SPE column of proteins and chaotropic agents,[0294]5. dry the SPE column to remove any residual organic solvents,[0295]6. elute the nucleic acids,[0296]7. mix the eluate with PCR master mix,[0297]8. thermally cycle the PCR mixture to amplify the target gene,[0298]9. and detect the resultant amplicon via an end-point fluorescence measurement.
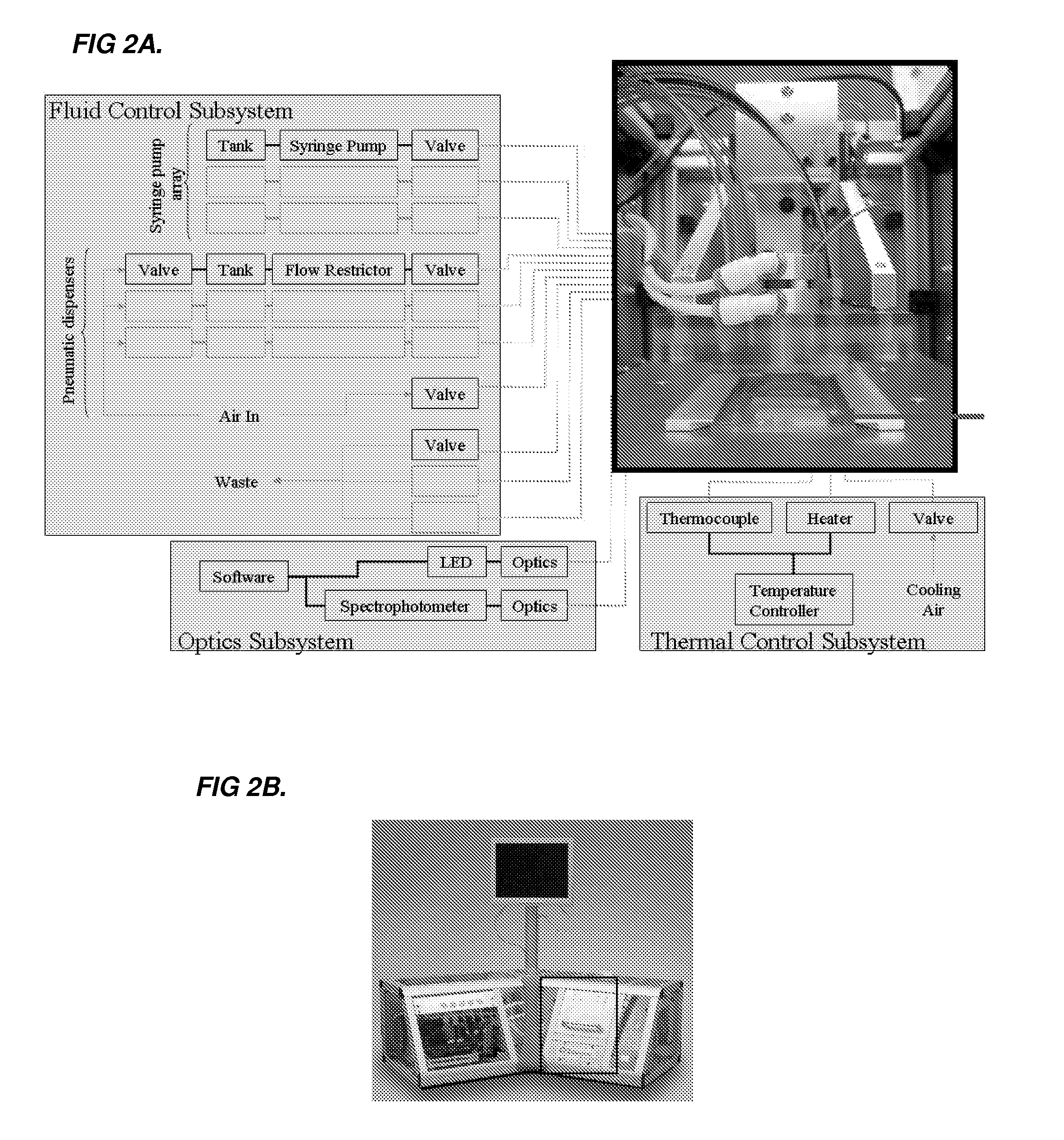
[0299]The system includes two major components: a disposable single-use plastic chip and an instrument which houses all active components to truly make the disposable low cost. To further minimize the cost of the chip, the microfluidic cha...
example 2
[0307]Bacterial detection: To demonstrate the utility of the fully functional chip for detection of bacteria, the inventors conducted a series of experiments with different numbers of input B. subtilis cells (1.25×106, 6.25×106, or 12.5×106 cells). For each chip, the inventors first ran a full-process negative control (water input) followed by the full process with the B. subtilis cells. The inventors present the optical detection data as the difference between the signal with B. subtilis and the same chip's negative control in FIG. 13A. After the on-chip detection was completed, the inventors collected the contents of the detection well and analyzed the sample by gel electrophoresis to confirm the presence of the target amplicon (FIG. 13B).
[0308]All three concentrations of B. subtilis were detected above the negative signal demonstrating that the system can successfully lyse the bacteria and isolate the nucleic acids, PCR amplify the target, and detect the presence of the amplicon ...
example 3
[0309]The main contributions described in this article are the design, implementation and demonstration of an end-to-end lab-on-a-chip system for the detection of bacteria that is truly low cost to manufacture. The field of microfluidic total analysis systems promises low-cost systems by miniaturizing and automating traditionally labor and reagent intensive processes. However, many of the innovations are complicated and expensive to manufacture and / or made of materials that are not robust for commercial application. To minimize the complexity and cost of the disposable component, the inventors designed a completely passive chip in a planar format that can be injection molded. The inventors have developed the injection molding process for this chip and will present the manufacturing methodology elsewhere. To achieve the full integration and automation of the laboratory steps required (sample and reagent introduction, mixing, nucleic acid isolation, PCR and optical detection), the inv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com