Quinoline derivative-containing pharmaceutical composition
a technology of pharmaceutical compositions and derivatives, which is applied in the direction of drug compositions, inorganic non-active ingredients, biocides, etc., can solve the problems of delayed dissolution of quinoline derivatives (i) and (i) of pharmaceutical compositions that are active ingredients, and achieve excellent absorption into the living body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0044]The present invention will be described in more detail below with reference to Examples, but is not limited to the Examples.
examples 1 to 3
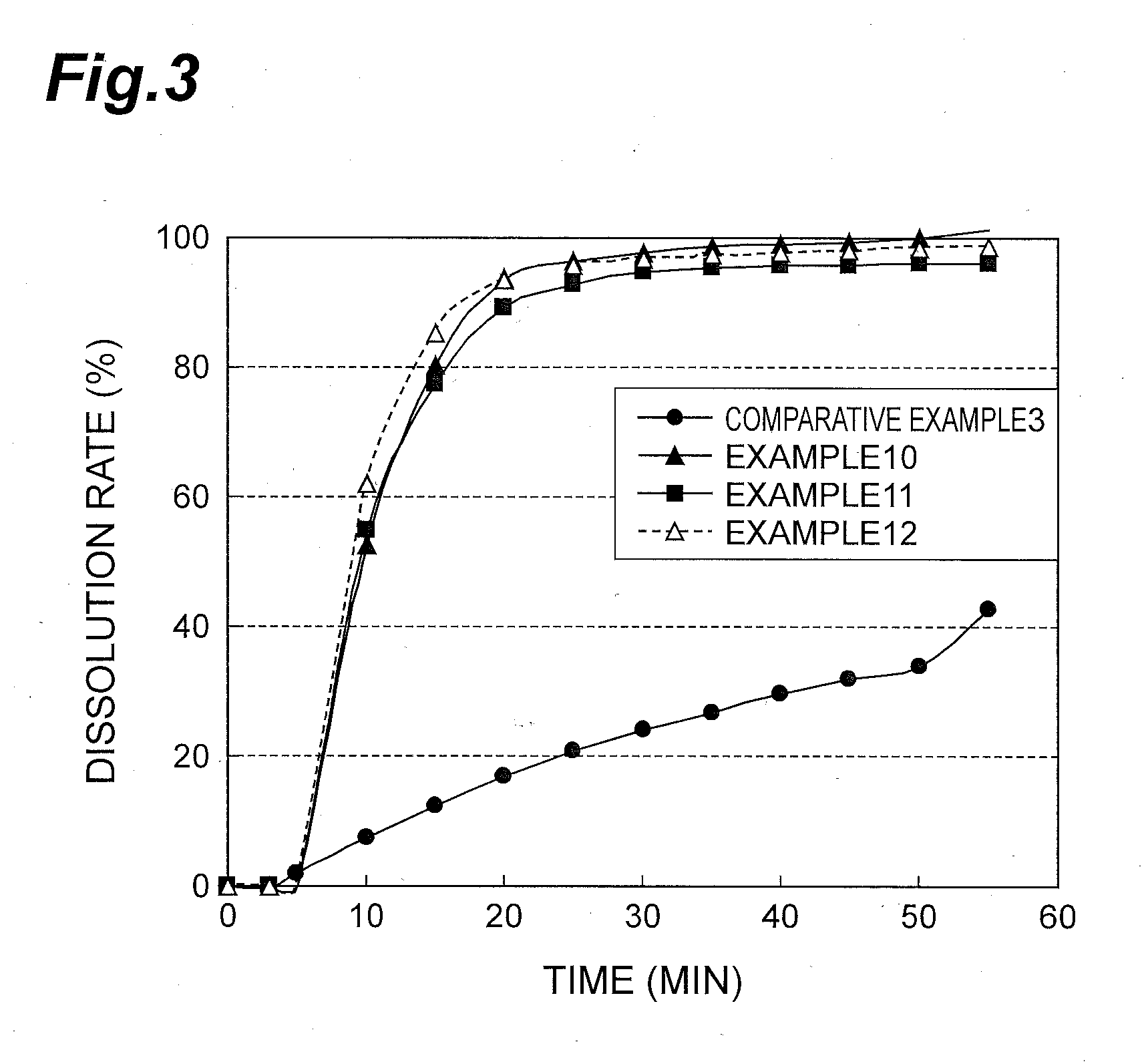
[0045]Wet granulation was performed with purified water as a solvent using a high-shear granulator (apparatus name: FM-VG-10, manufactured by Powrex Corporation) with the C form crystal of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide methanesulfonate (hereinafter referred to as compound A), D-mannitol (trade name: Mannitol, Merck), precipitated calcium carbonate (trade name: Whiton F, Shiraishi Calcium), hydroxypropylcellulose (HPC-L, Nippon Soda), low-substituted hydroxypropylcellulose (trade name: L-HPC (LH-21), Shin-Etsu Chemical) and microcrystalline cellulose (trade name: Ceolus PH-101, Asahi Kasei Chemicals) according to the formulation proportions in Table 1. The granules of which a moisture content was reduced to be less than 2% by further drying were sized using a screen mill (apparatus name: Power Mill P-04S, manufactured by Showa Giken KK) so that their granule diameters were less than 1 mm. Then, microcrystalline cellulose (trade...
examples 4 to 9
, Comparative Examples 1 to 2
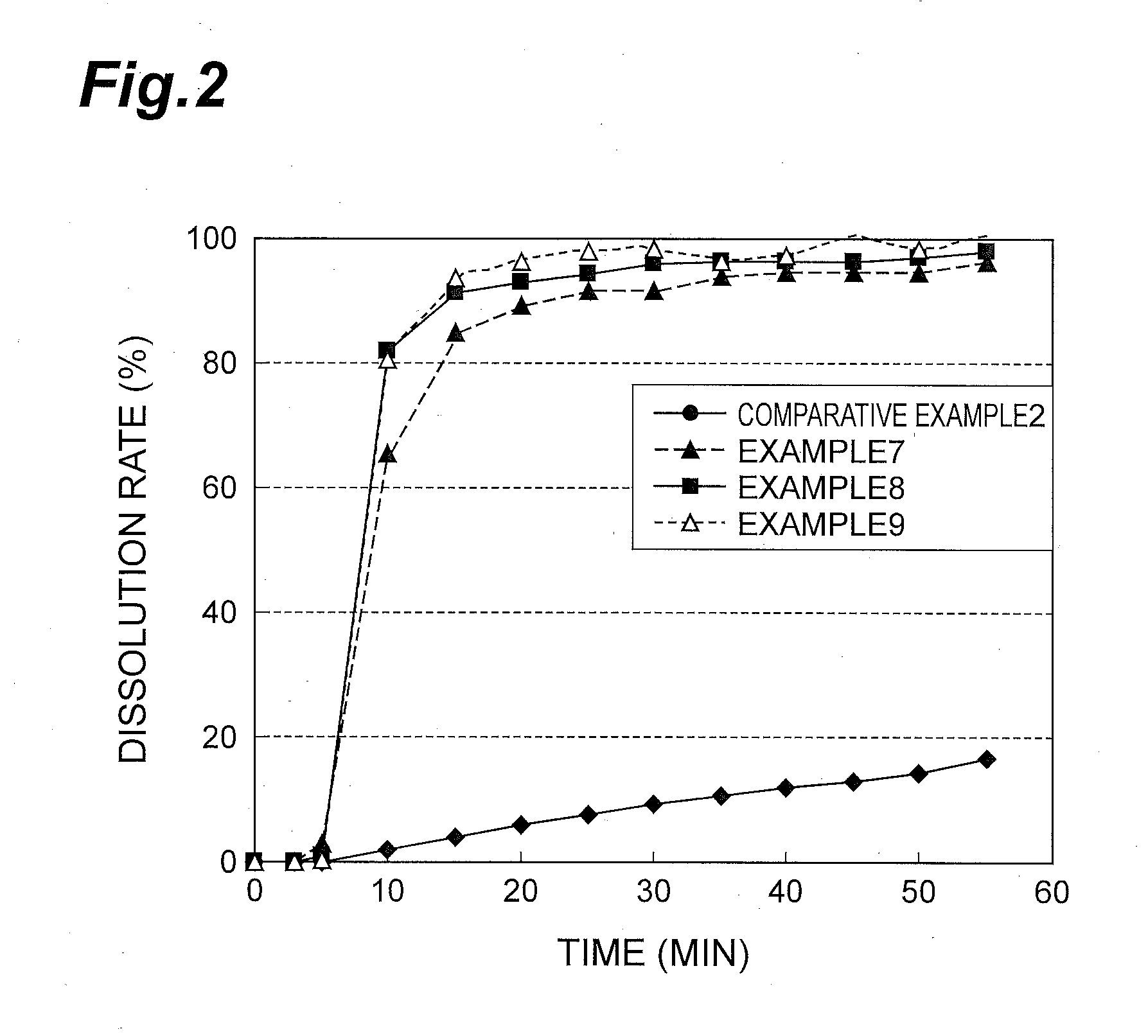
[0046]The compound A, precipitated calcium carbonate, low-substituted hydroxypropylcellulose, D-mannitol and talc were thoroughly mixed using a mortar and a pestle according to the formulation proportions in Table 2 and Table 3. Hard capsules size #3 were filled with 100 mg of the resultant mixtures to prepare capsules in Examples 4 to 9. Capsules in Comparative Examples 1 to 2, which contained no precipitated calcium carbonate, were also prepared by the same method.
TABLE 2Com.Ex. 1Ex. 4Ex. 5Ex. 6Compound A5555Precipitated calcium carbonate051020Low-substituted30252010hydroxypropylcelluloseD-Mannitol62626262Talc3333Total100100100100Unit: weight %
TABLE 3Com.Ex. 2Ex. 7Ex. 8Ex. 9Compound A20202020Precipitated calcium carbonate051020Low-substituted30252010hydroxypropylcelluloseD-Mannitol47474747Talc3333Total100100100100Unit: weight %
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com