Pharmaceutical composition of peptide drug and enzyme-inhibition compounds
a technology of enzyme inhibitors and pharmaceutical compositions, applied in the field of oral drug delivery vehicles, can solve the problems of limited hydrophilic molecules, inability to easily diffuse hydrophilic drugs across cells, and inability to transport hydrophilic molecules via paracellular pathways, and achieve the effect of reducing intestinal permeability and improving intestinal epithelial barrier function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example no.1
EXAMPLE NO. 1
[0074]Materials and Methods of Nanoparticles Preparation
[0075]CS (MW ˜2.8×105) with a degree of deacetylation of approximately 85% was acquired from Challenge Bioproducts Co. (Taichung, Taiwan). Acetic acid, cellulase (1.92 units / mg), fluorescein isothiocyanate (FITC), phosphate buffered saline (PBS), periodic acid, sodium acetate, formaldehyde, bismuth subnitrate, and Hanks' balanced salt solution (HBSS) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Ethanol absolute anhydrous and potassium sodium tartrate were obtained from Merck (Darmstadt, Germany). Non-essential amino acid (NEAA) solution, fetal bovine serum (FBS), gentamicin and trypsin-EDTA were acquired from Gibco (Grand Island, N.Y.). Eagle's minimal essential medium (MEM) was purchased from Bio West (Nuaille, France). All other chemicals and reagents used were of analytical grade.
example no.2
[0076]Depolymerization of CS by Enzymatic Hydrolysis
[0077]Regular CS was treated with enzyme (cellulase) to produce low-MW CS according to a method described by Qin et al. with some modifications (Food Chem. 2004;84:107-115). A solution of CS (20 g / l) was prepared by dissolving CS in 2% acetic acid. Care was taken to ensure the total solubility of CS. Then, the CS solution was introduced into a vessel and adjusted to the desired pH 5.0 with 2N aqueous NaOH. Subsequently, cellulase (0.1 g) was added into the CS solution (100 ml) and continuously stirred at 37° C. for 12 hours. Afterward, the depolymerized CS was precipitated with aqueous NaOH at pH 7.0-7.2 and the precipitated CS was washed three times with deionized water. The resulting low-MW CS was lyophilized in a freeze dryer (Eyela Co. Ltd, Tokyo, Japan).
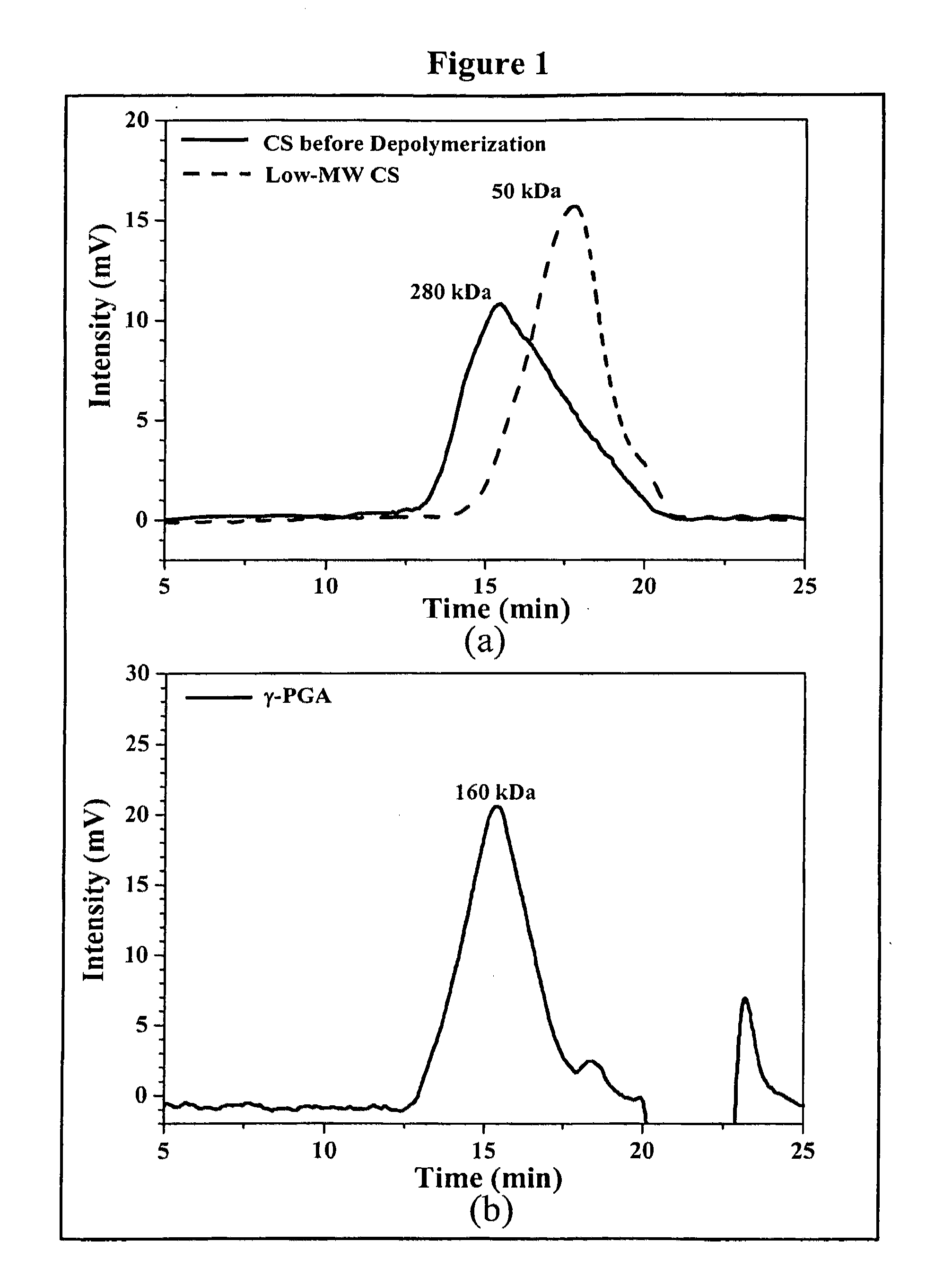
[0078]The average molecular weight of the depolymerized CS was determined by a gel permeation chromatography (GPC) system equipped with a series of PL aquagel-OH columns (one G...
example no.3
EXAMPLE NO. 3
[0083]Preparation of the CS-γ-PGA Nanoparticles
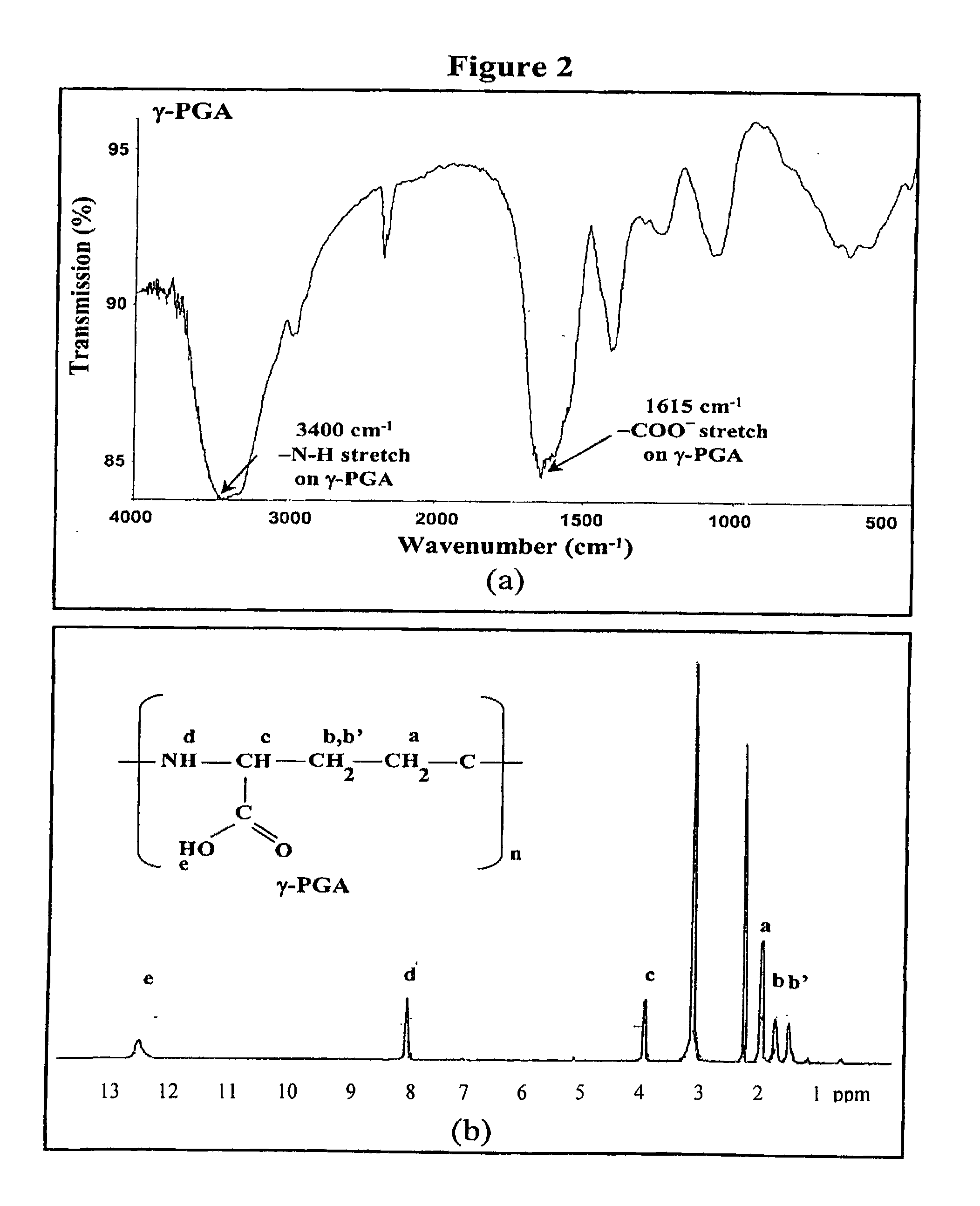
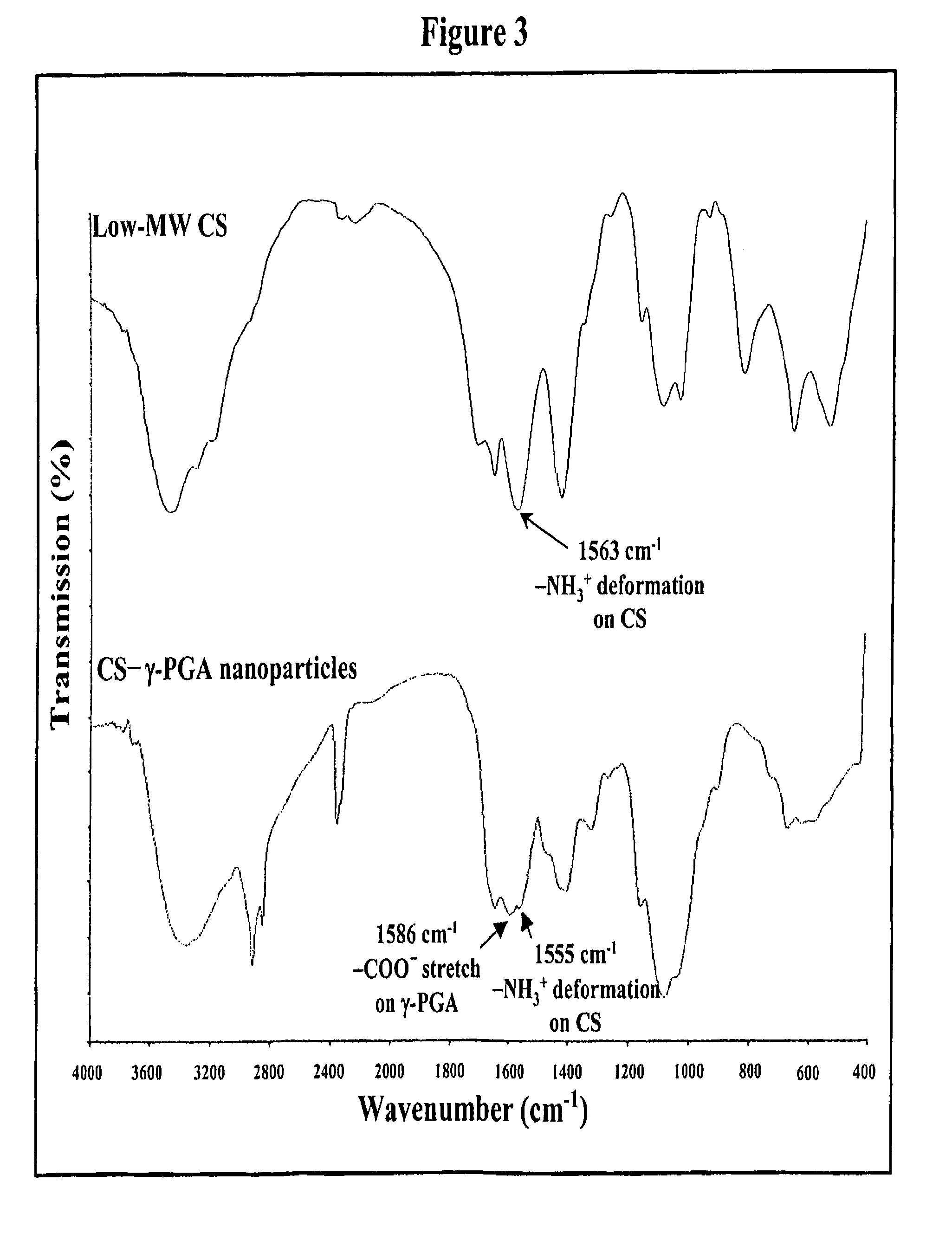
[0084]Nanoparticles were obtained upon addition of γ-PGA aqueous solution (pH 7.4, 2 ml), using a pipette (0.5-5 ml, PLASTIBRAND®, BrandTech Scientific Inc., Germany), into a low-MW CS aqueous solution (pH 6.0, 10 ml) at varying concentrations (0.01%, 0.05%, 0.10%, 0.15%, or 0.20% by w / v) under magnetic stirring at room temperature. Nanoparticles were collected by ultracentrifugation at 38,000 rpm for 1 hour. Supernatants were discarded and nanoparticles were resuspended in deionized water for further studies. The nanoparticles thus obtained via the simple and mild ionic-gelation method described herein show typical characteristics in a spheroidal configuration with a particle size of between about 50 to 400 nm, a positive surface charge and a narrow polydispersity index. FT-IR was used to analyze peak variations of amino groups of low-MW CS and carboxylic acid salts of γ-PGA in the CS-γ-PGA nanoparticles.
[0085]As stated, n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| MW | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com