Composition comprising cell and biocompatible polymer
a biocompatible polymer and cell technology, applied in the direction of genetically modified cells, skeletal/connective tissue cells, peptide/protein ingredients, etc., can solve the problems of difficult treatment, long time to obtain, and poor regenerative power of central nervous system tissues, so as to improve the survival rate of cells and suppress the outflow of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Biocompatible Polymer Particles
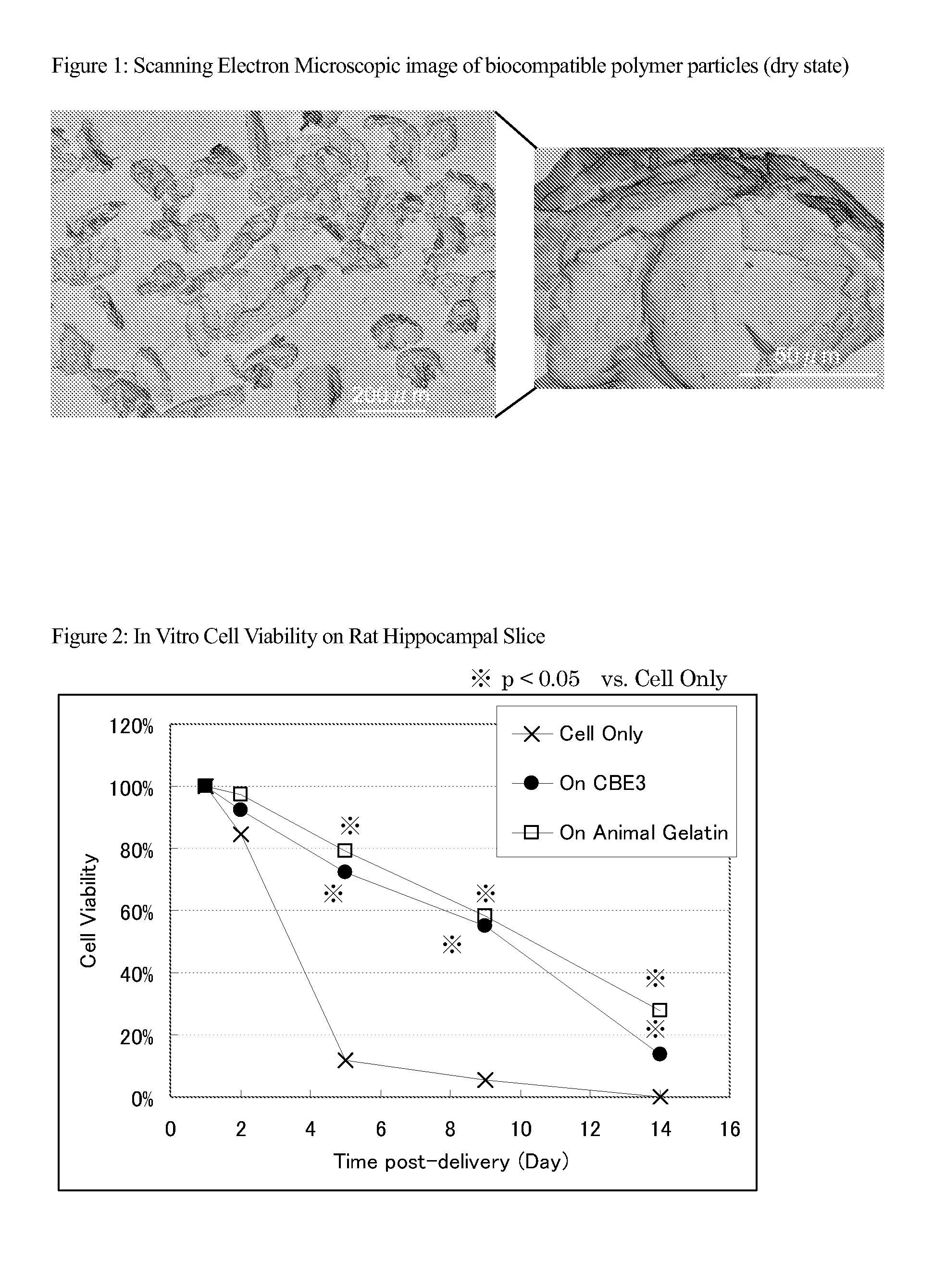
[0128]A 18 ml of 3% Glutaraldehyde solution was added into 162 ml of 10% (w / v) CBE3 aqueous solution. The mixture was left at 4 oC for 18 h to make a hydro gel. The hydro gel was freeze dried and milled to make small pieces of CBE3 particles. The photographs of the obtained CBE3 particles are shown in FIG. 1.
example 2
In Vitro Cell Viability on Rat Hippocampal Slice
[0129]10% (v / v) of CBE3 particles or animal gelatin particles were suspended in a medium (α-MEM; 10% FBS; 1% PS). Thereafter, GFP-labeled Notch intracellular gene induced bone marrow derived cells (SB623 manufactured by Sanbio) were suspended in each solution. The thus produced cell suspension was added onto the hippocampal section of a P9 rat placed on a 12-well plastic plate.
[0130]The section was then incubated in a CO2 incubator at 37° C. One, two, five, nine and fourteen days after initiation of the incubation, cells expressing GFP on the section were observed under a fluorescent microscope, and the number of such cells was counted. The cells that expressed GFP were considered to be surviving cells. Thus, the survival rate of the cells was evaluated as a ratio with respect to the number of cells 1 day after initiation of the inoculation. The results are shown in FIG. 2.
[0131]The survival rate of the cells was improved by the effect...
example 3
In Vitro Cell Viability in Ultra Low Attachment Culture Plate
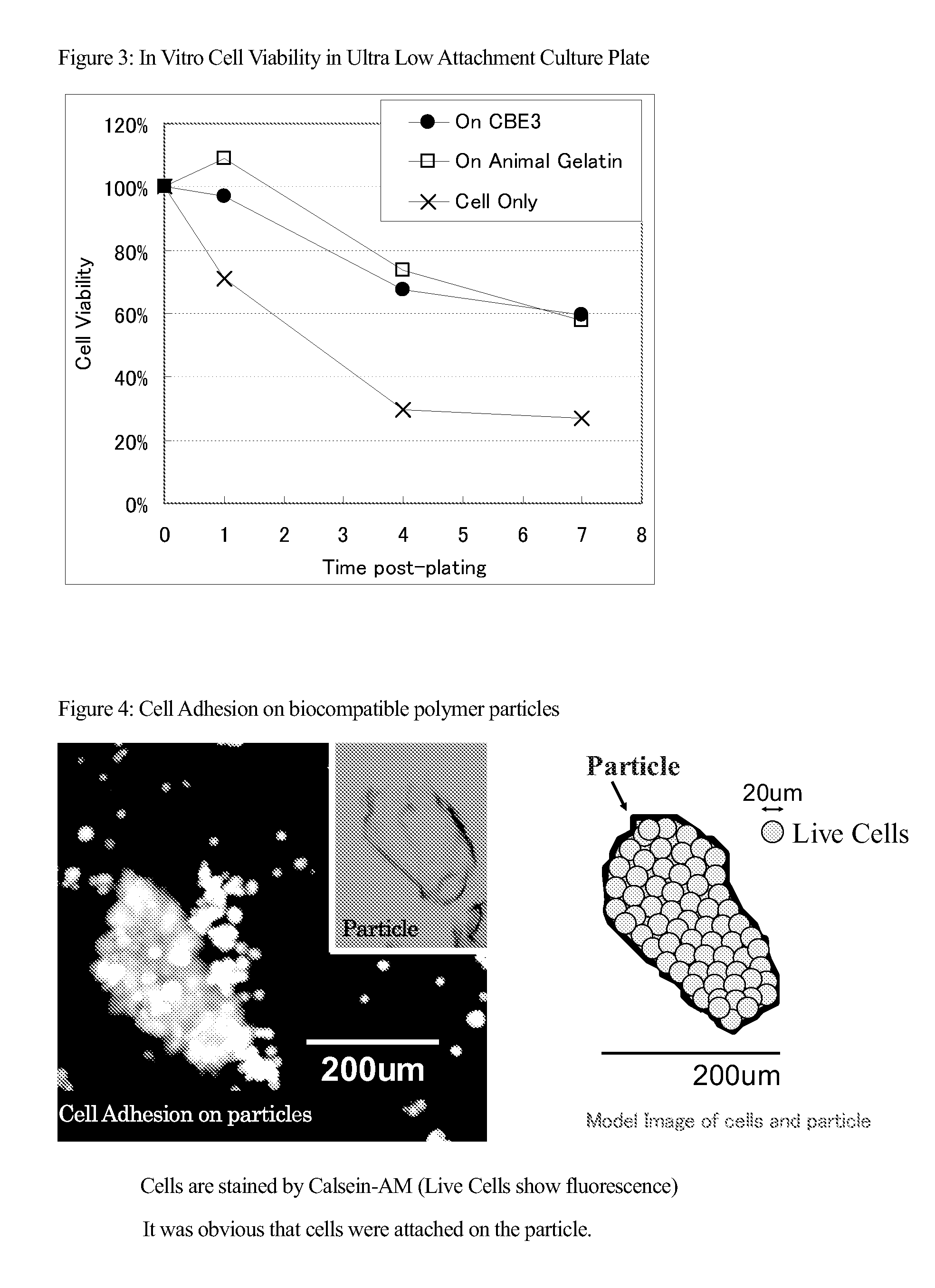
[0132]0.01 cm3 / well of CBE3 and animal gelatin particles in 500 ul of α-MEM are placed in a Ultra Low Attachment Culture Dish (manufactured by Corning). Thereafter, 50,000 of Notch intracellular gene induced bone marrow derived cells (SB623 manufactured by Sanbio) were plated and incubated in a CO2 incubator at 37° C. 2 hours and one, four, and seven days after initiation of the incubation, samples are filtered by a cell strainer (mesh size 40 um) and DNA is extracted from recovered samples by dissolving in papain solution (2.5 mU / ml papain, 5 mM L-cystein, 5 mM EDTA) at 60 oC overnight. By using standard curve, number of cells on particles was counted. Because live cells adhere on particles and dead cells do not, the survival rate of the cells was evaluated as a ratio with respect to the number of cells 0 day (2 hours) after plating.
[0133]As shown in FIG. 3, the survival rate of the cells was improved by CBE3 particles or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Adhesion strength | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com