Method of operating a direct dme fuel cell system
a fuel cell and direct technology, applied in the direction of fuel cells, fuel cell auxiliaries, electrochemical generators, etc., can solve the problems of methanol toxic, loss of voltage and overall performance, and limited use of nafion® membranes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Membrane Electrode Assembly
[0056]The electrodes, a PtRu / C anode and a Pt / C cathode, were produced by tape-casting. A carbon paper anode was loaded by use of a 60% 2:1 Pt—Ru catalyst powder (Platinum, nominally 40%, Ruthenium, nominally 20% on carbon black; Alfa Aesar GmbH & Co KG) added to a PBI solution (4% wt in N,N-dimethylacetamide, DMAc) using amounts resulting in a loading of 1.5 mg Pt—Ru per cm2 and 0.3 mg PBI per cm2. A carbon paper cathode was loaded by use of a 38.6% Pt catalyst (powder, prepared by reduction of Pt from a hexacloroplatinate solution) added to a PBI solution (4% wt in N,N-dimethylacetamide, DMAc) using amounts resulting in a loading of 0.7 mg Pt per cm2 and 0.3 mg PBI per cm2. After drying, doping of PBI was performed using a phosphoric acid solution for establishing an acid doping level of 6.
[0057]The MEA was obtained by hotpressing the electrodes on either side of a phosphoric-acid-doped PBI membrane produced by solution casting (acid dopin...
example 2
Performance at Different Temperatures
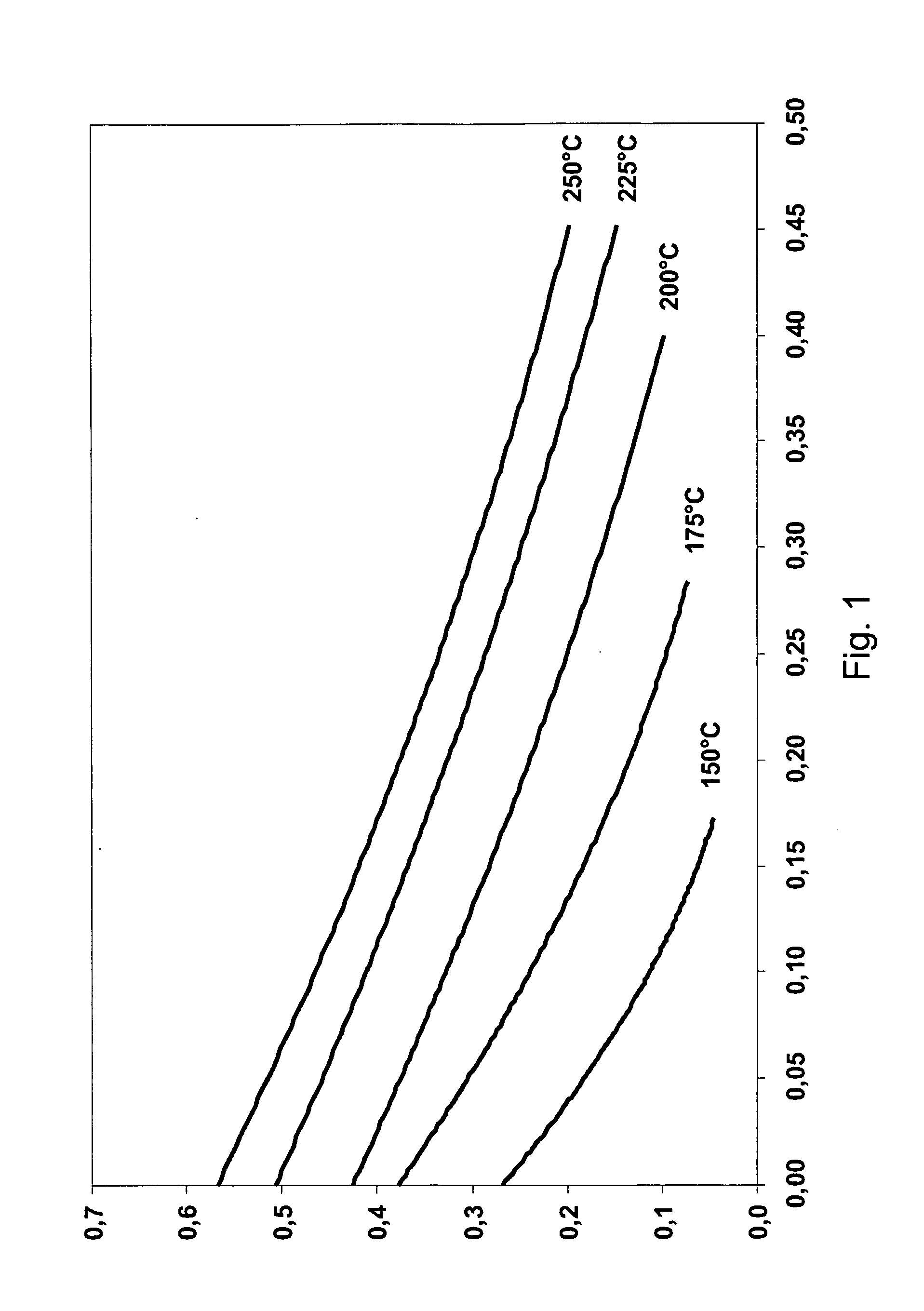
[0058]Polarisation curves were obtained at 150, 175, 200, 225 and 250° C. Peak power densities at different temperatures are shown in table 1.
TABLE 1Maximum power densityTemperature (° C.)(mW / cm2)1501217529200522257825096
[0059]At 200° C., the cell had the lowest ohmic resistance. At temperatures of 200° C. or above, the ohmic loss remains constant. After an operating time of one hour, the maximum power densities were further increased to values of about 100 mW / cm2 at 200° C. and at an equivalence ratio of between 1.1 and 1.5. Open circuit voltages (OCVs) were about 0.7 V.
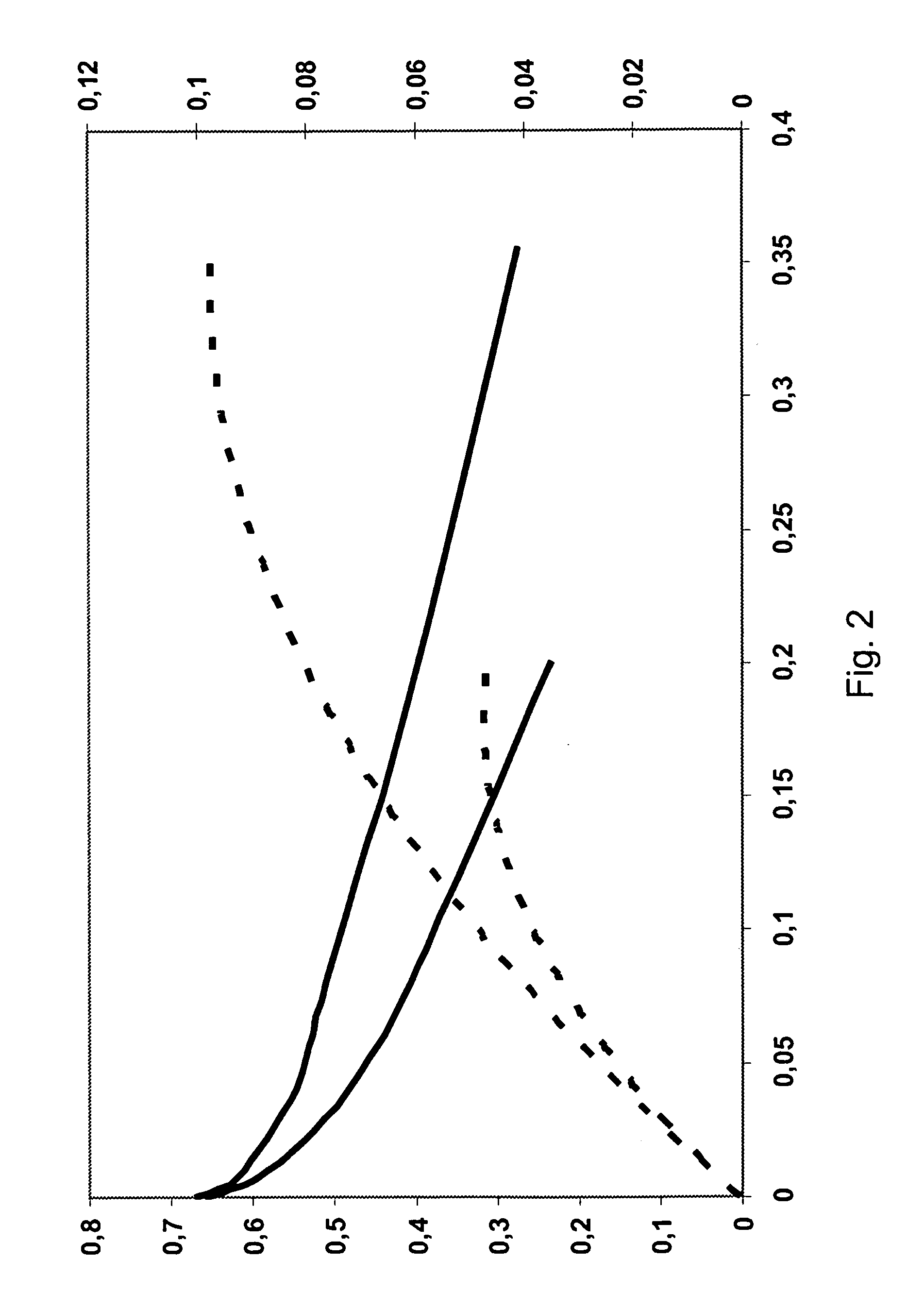
[0060]Exemplary polarisation curves are shown in FIGS. 1 and 2, where FIG. 1 shows results from an early experiment and FIG. 2 shows test results from a later experiment.
example 3
Membrane Crossover of DME and Methanol
[0061]Air was evacuated from a first gas chamber and to a second gas chamber separated from each other by a phosphoric-acid-doped PBI membrane (acid doping: 6, thickness: 50 μm). The resulting absolute pressure inside both chambers was below 20 mbar. Subsequently, either gaseous methanol or DME was injected into the first gas chamber. The starting pressure of DME was 4 bar, and the starting pressure for methanol was 6 bar. The rate of gas transport across the membrane was determined by recording the pressure build up in the second gas chamber over time.
[0062]The membrane permeabilities were calculated as 7.4*10−9 mol cm−1 s−1 bar−1 for DME and 10.4*10−9 mol cm−1 s−1 bar−1 for methanol. Thus, methanol diffuses through the membrane at a rate that is about 40% higher than the rate of DME diffusion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com