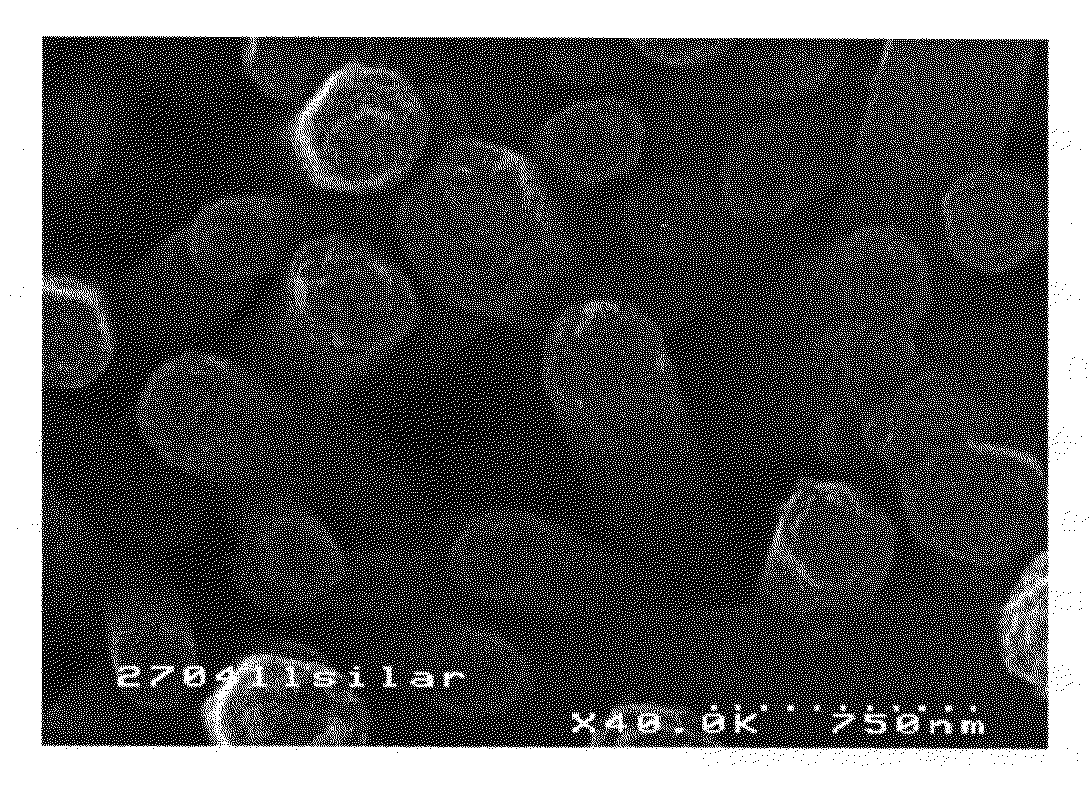Method for producing a film by cu2znsns4 silar
a technology of cu2znsns and silar, which is applied in the direction of liquid/solution decomposition chemical coating, instruments, transportation and packaging, etc., can solve the problems of difficult to obtain only czts, and it is not possible to achieve uniform films of controlled thickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0058]The method according to the invention was used to form a deposit of CZTS by the SILAR technique on ZnO nanowires. The duration of immersion in the cationic then anionic solutions was 20 seconds each, and the duration of immersion for rinsing in a bath of deionized water was 20 seconds
TABLE 1Description of the different methods used for deposition of CZTSby the SILAR technique.AnionicMethodCationic solutionsolutionP2 5 mM CuSO4 5 mM SnSO410 mM ZnSO410 mM Na2SP310 mM CuSO4 5 mM SnSO410 mM ZnSO410 mM Na2SP410 mM CuSO4 5 mM SnSO4 5 mM ZnSO410 mM Na2SP510 mM CuSO410 mM SnSO410 mM ZnSO410 mM Na2S
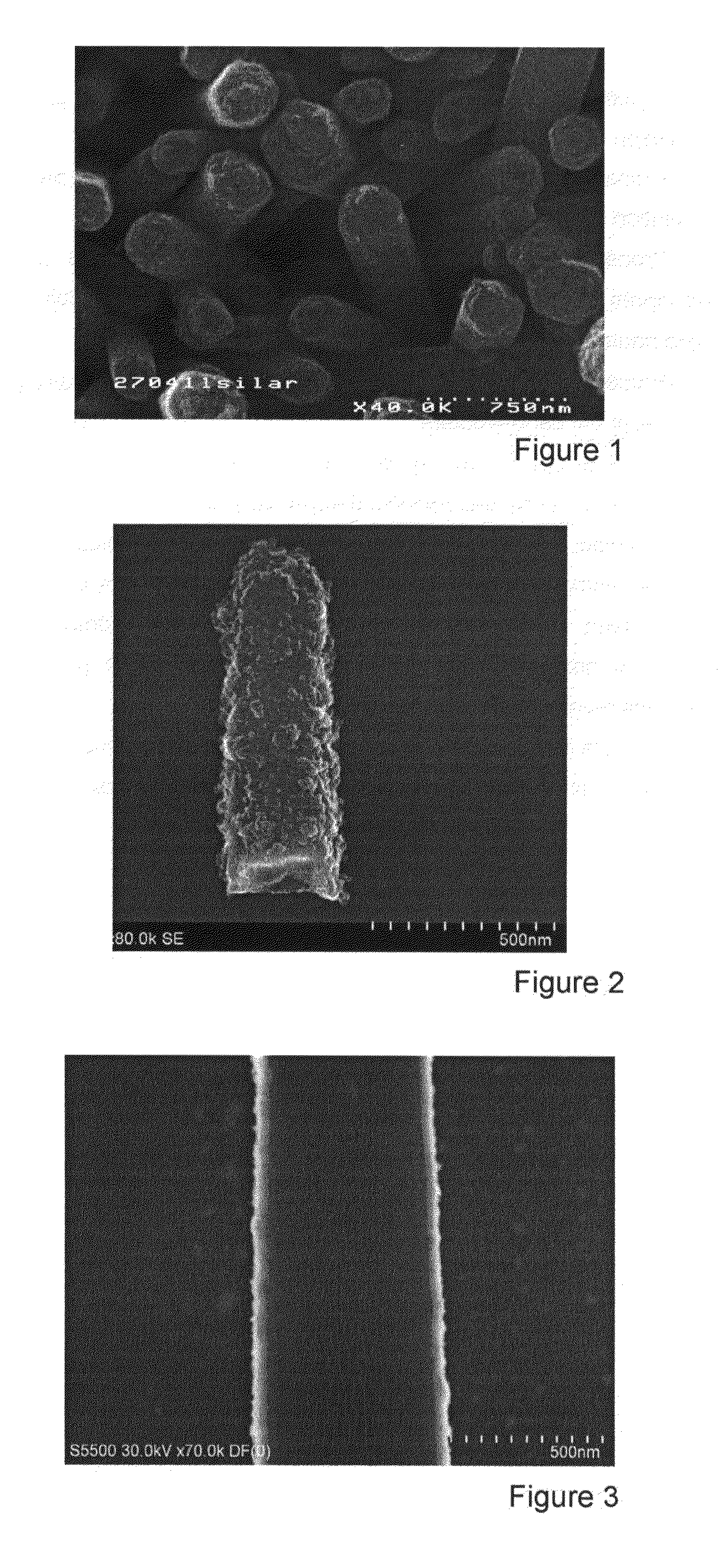
[0059]FIG. 1: SEM image from above of Cu2ZnSnS4 deposited by SILAR on ZnO using method P5.
[0060]FIGS. 2 and 3: STEM image of a structure comprising a ZnO nanowire core and Cu2ZnSnS4 shell using method P5.
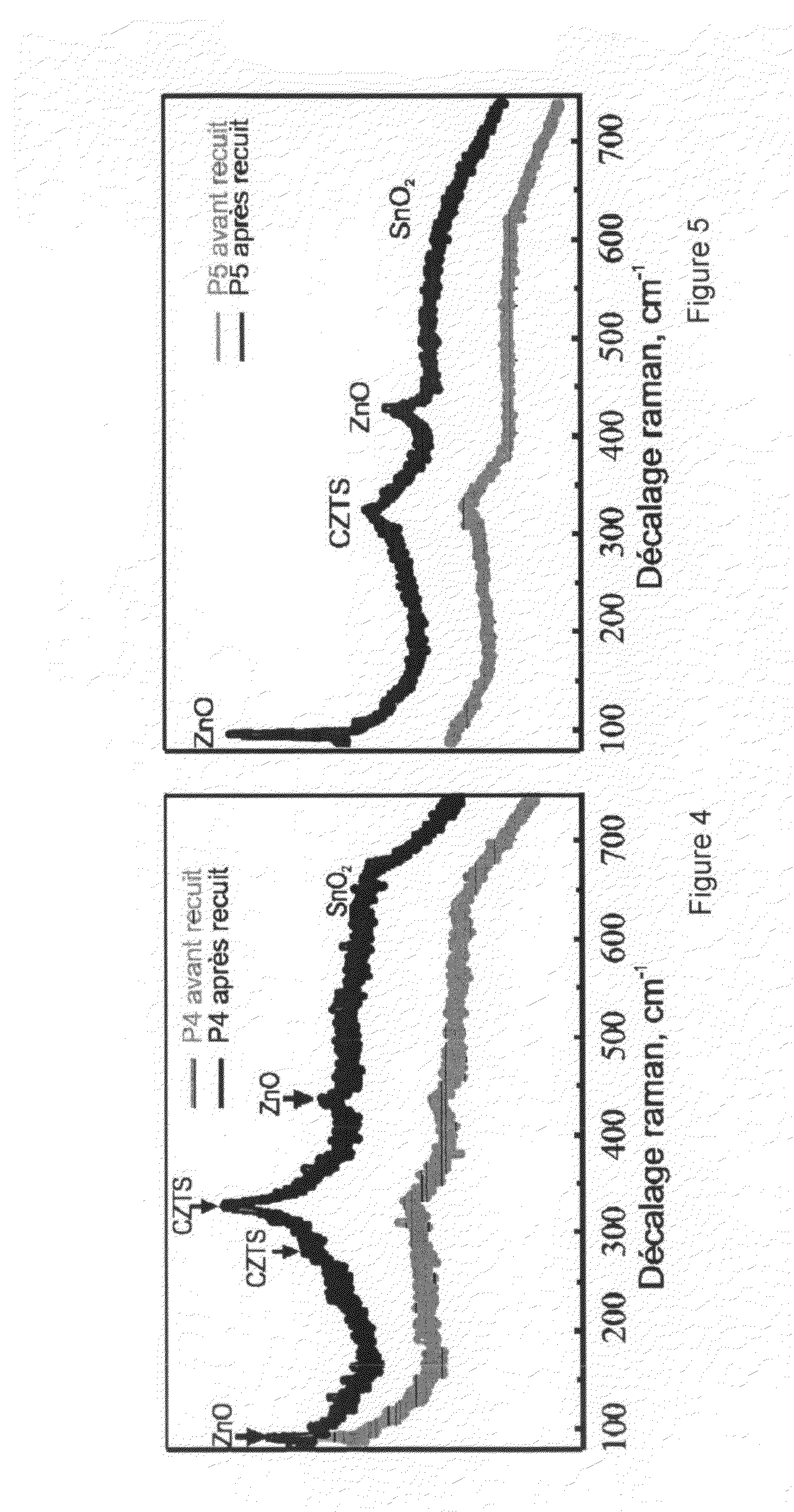
[0061]Analyses by the Raman technique were carried out on the CZTS films obtained by the methods of Table 1.
[0062]FIGS. 4 and 5
[0063]These two spectra correspond to the CZTS films obtained r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com