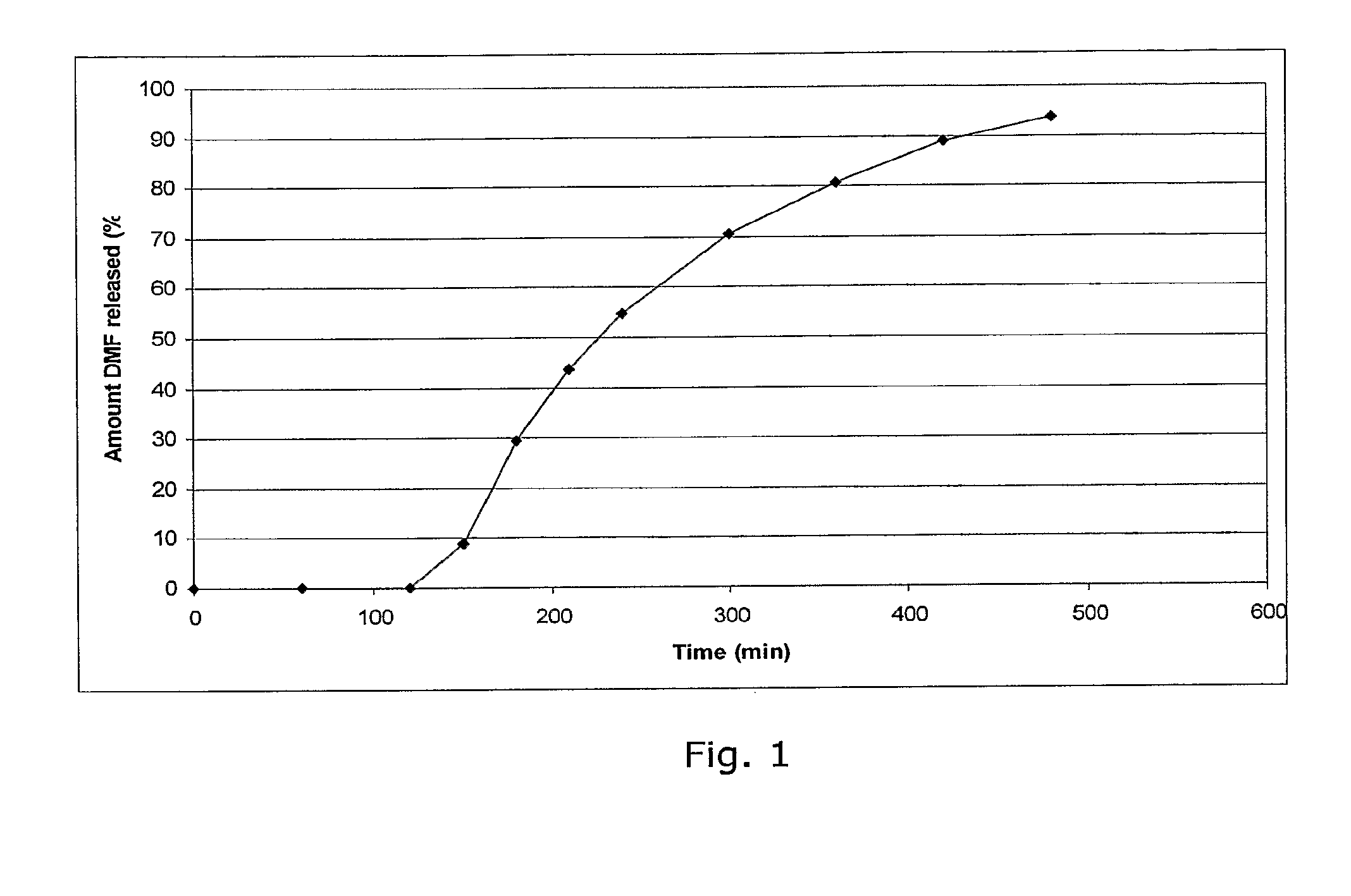
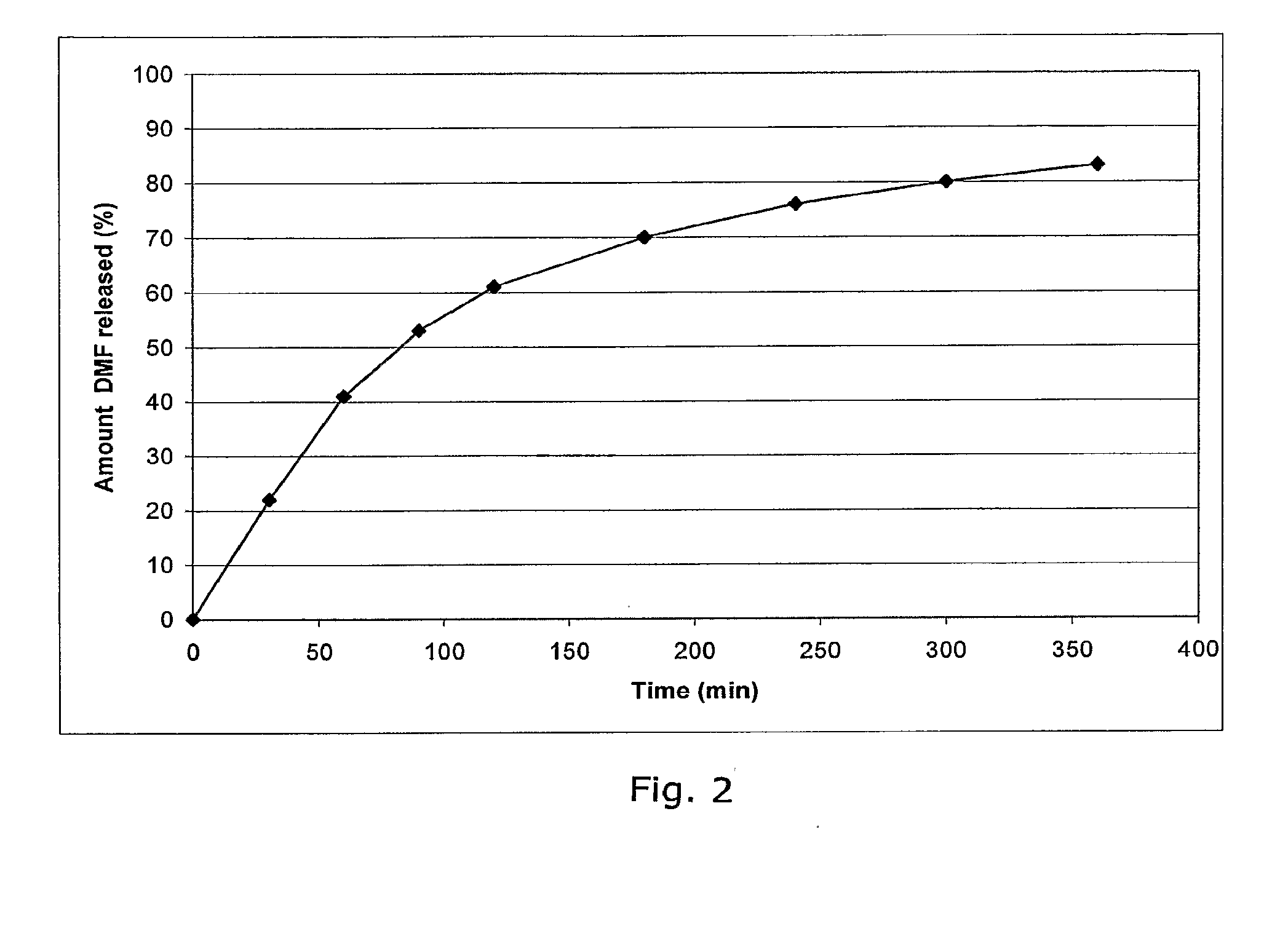
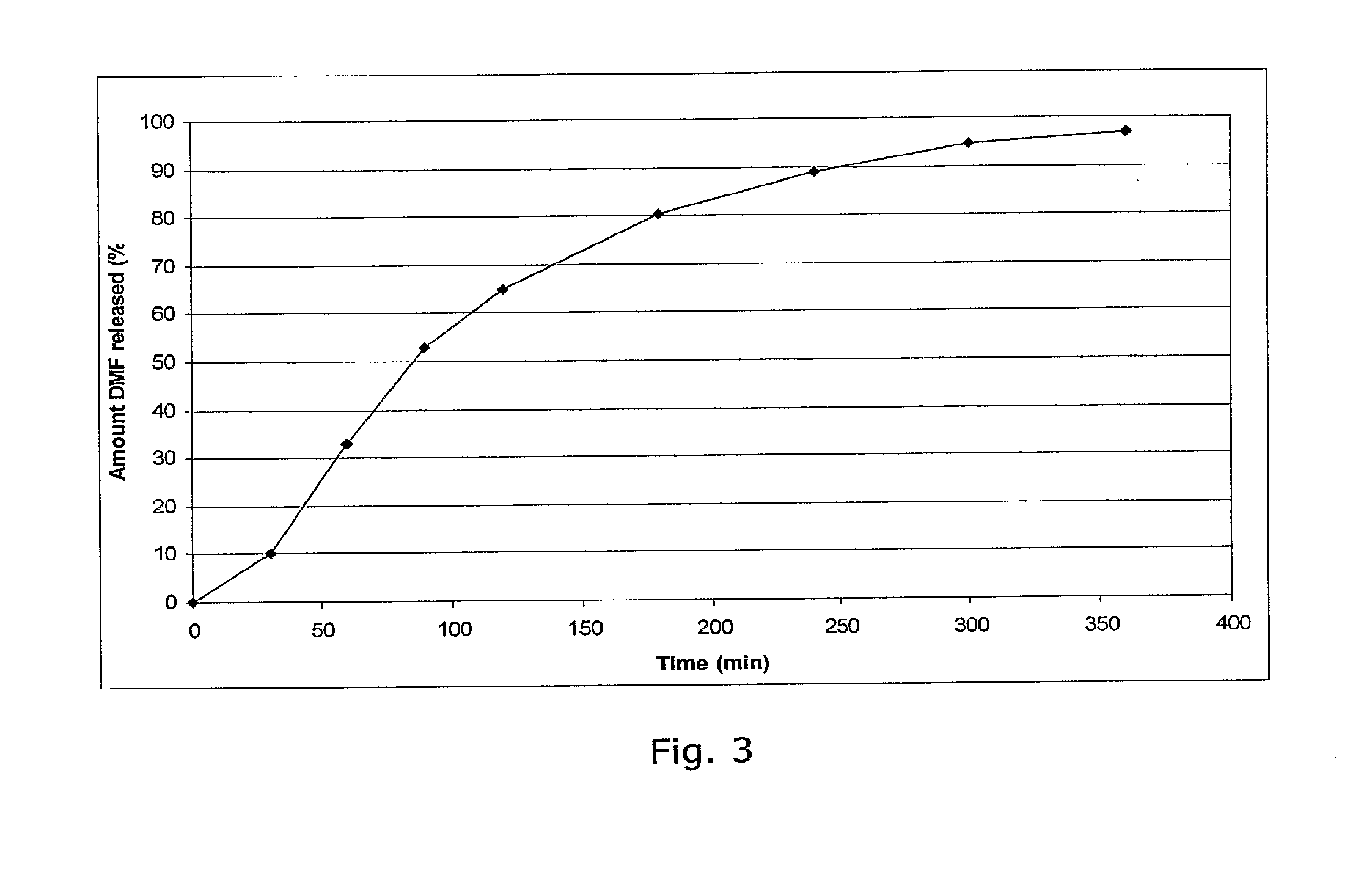
Controlled release pharmaceutical compositions comprising a fumaric acid ester
a technology of controlled release and pharmaceutical compositions, which is applied in the direction of botany apparatus and processes, pharmaceuticals, applications, etc., can solve the problems that fumarate therapy like e.g. fumaderm® often gives rise to gastro-intestinal side effects, and achieves the effects of reducing dependence, prolonging time period, and reducing interindividual and/or intraindividual variation in plasma profiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Tablets
[0169]200 g granules are mixed with 150 g microcrystalline cellulose (e.g. Avicel® 102), 97.5 g lactose (e.g. Tablettose®), 10 g sodium carboxymethylcellulose (e.g. Ac-Di-Sol®) and 25 g starch for 30 min. Then 10 g magnesium stearate and 7.5 g amorphous silicium dioxide (e.g. Aerosil® 200) is added and the powder mixture is mixed for 5 min.
[0170]This powder mixture is compressed to tablets in tabletting equipment (tablet diameter 10 mm, surface about 280-300 mm2). The tablets are enteric coated in a pan-coating or in a fluid-bed coating process as described in Example 4.
example 2
Preparation of Tablets
[0171]200 g micro-crystals are mixed with 150 g microcrystalline cellulose (e.g. Avicel® 102), 130 g lactose (e.g. Tablettose®), 10 g of sodium carboxymethylcellulose (e.g. Ac-Di-Son and 25 mg starch for 30 min. Then 10 g magnesium stearate and 7.5 g of amorphous silicium dioxide is added and the powder mixture is mixed for 5 min. This powder mixture is compressed to tablets in tabletting equipment (tablet diameter 10 mm, surface about 280-300 mm2). The tablets are enteric coated in a pan-coating or in a fluid-bed coating process as described in Example 4.
example 3
Preparation of Capsules
[0172]Granules or micro-crystals are filled in HPMC capsules and these capsules are enteric coated as described in the following. In a pan coater Eudragit® L30D-55 is sprayed at drying temperatures of 60° C. to 80° C. onto the capsules in an amount of 20 mg polymeric material per mm2. Pigments and talc are added in an appropriate amount.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com