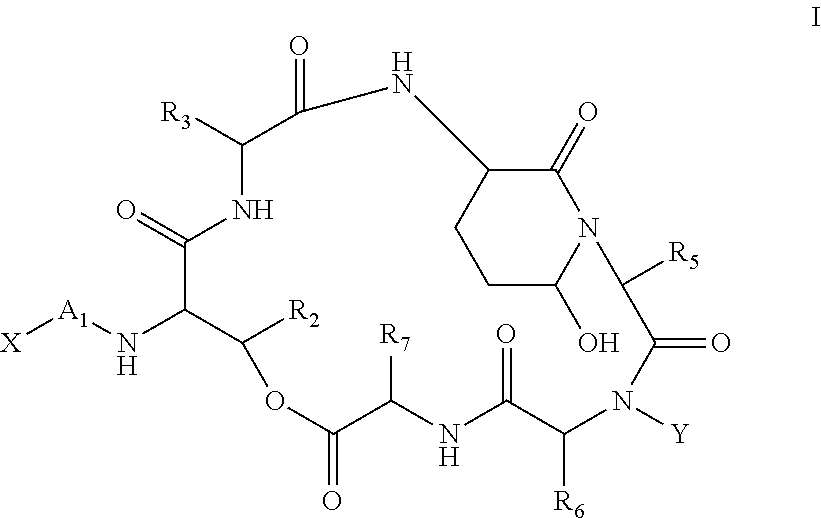
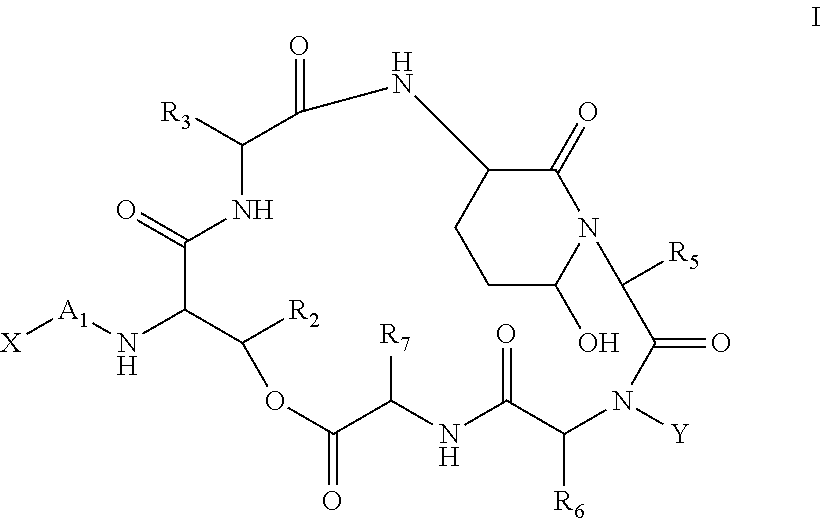
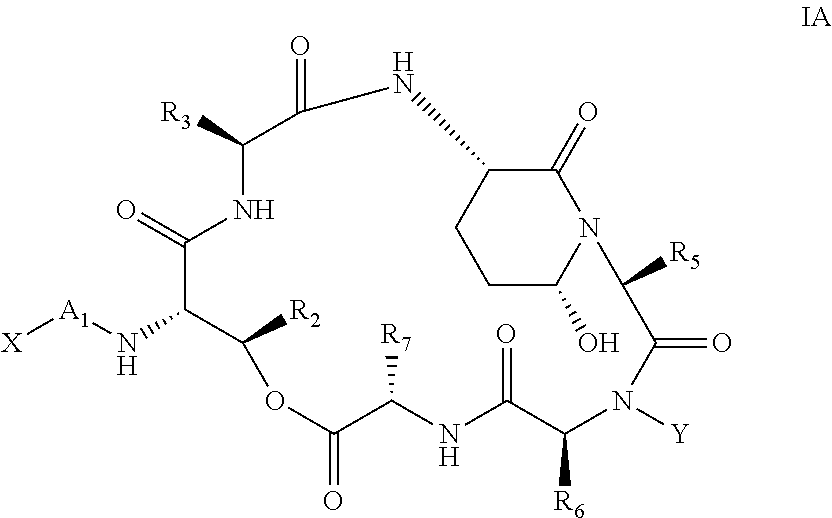
Aldehyde acetal based processes for the manufacture of macrocyclic depsipeptides and new intermediates
a technology of macrocyclic depsipeptides and aldehyde acetal, which is applied in the direction of peptides, immunoglobulins, peptide/protein ingredients, etc., can solve the problems of high cost, difficult preparation of ethers or analogues thereof, and low yield of fermentation with regard to any single of these compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of Compound a Using an Acyclic Acetal Protecting Group: Employing of the Dibenzyl Acetale
[0216]The following reaction scheme shows the use of compound 9 as an alternative to Compound 7 in Example 1:
[0217]In detail, the corresponding precursors and the reactions in the scheme are realized as follows:
2 A) Synthesis of the dibenzyl-acetale synthon (Compound 17)
[0218]Compound 17 was prepared according to the synthesis scheme shown below:
2 B) Synthesis of Compound 12
[0219]In a 3 L-reactor under nitrogen at 0° C., dried acrolein (105.3 g, 1.78 mol) in methylene chloride (1.15 kg) was introduced Trimethylbromosilane (281.7 g, 1.78 mol) was added dropwise over 30 min with a dropping funnel, keeping the temperature below 5° C. After 1 h 30 min of stirring, benzyl alcohol (311.1 g, 2.85 mol) was added dropwise over 45 min, and the mixture was stirred at 0° C. for 16 h. To the orange solution, pyridine (29 g, 0.36 mol), acetic anhydride (38 g, 0.36 mol) and DMAP (4.4 g, 0.04 mol) wer...
example 3
Shift of Equilibrium
[0239]A possible side reaction of the acetal cleavage and formation of ahp may result in the dehydrate form of Compound A. This can be converted easily (back) into Compound A using a simple procedure for hydration of the dehydrate form depicted in the following reaction scheme:
[0240]This allows to improve the yield of Compound A in any type of synthesis (be it chemical as in the present disclosure or by use of fermentation as in WO2009 / 024527).
[0241]For example, during cleavage of acid sensitive protecting groups in a compound comprising the ahp-subunit, e.g. Compound A in the Scheme before Example 1, the formation of large amounts of the corresponding dehydrated byproduct (Compound A-Dehydrate) is observed. This byproduct is usually separated e.g. by chromatography and disposed. This leads to loss of valuable product and to low yield for this step. For example, if the deprotection product(s) of Compound 8 are subjected to acidic conditions to cleave the trityl- ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com