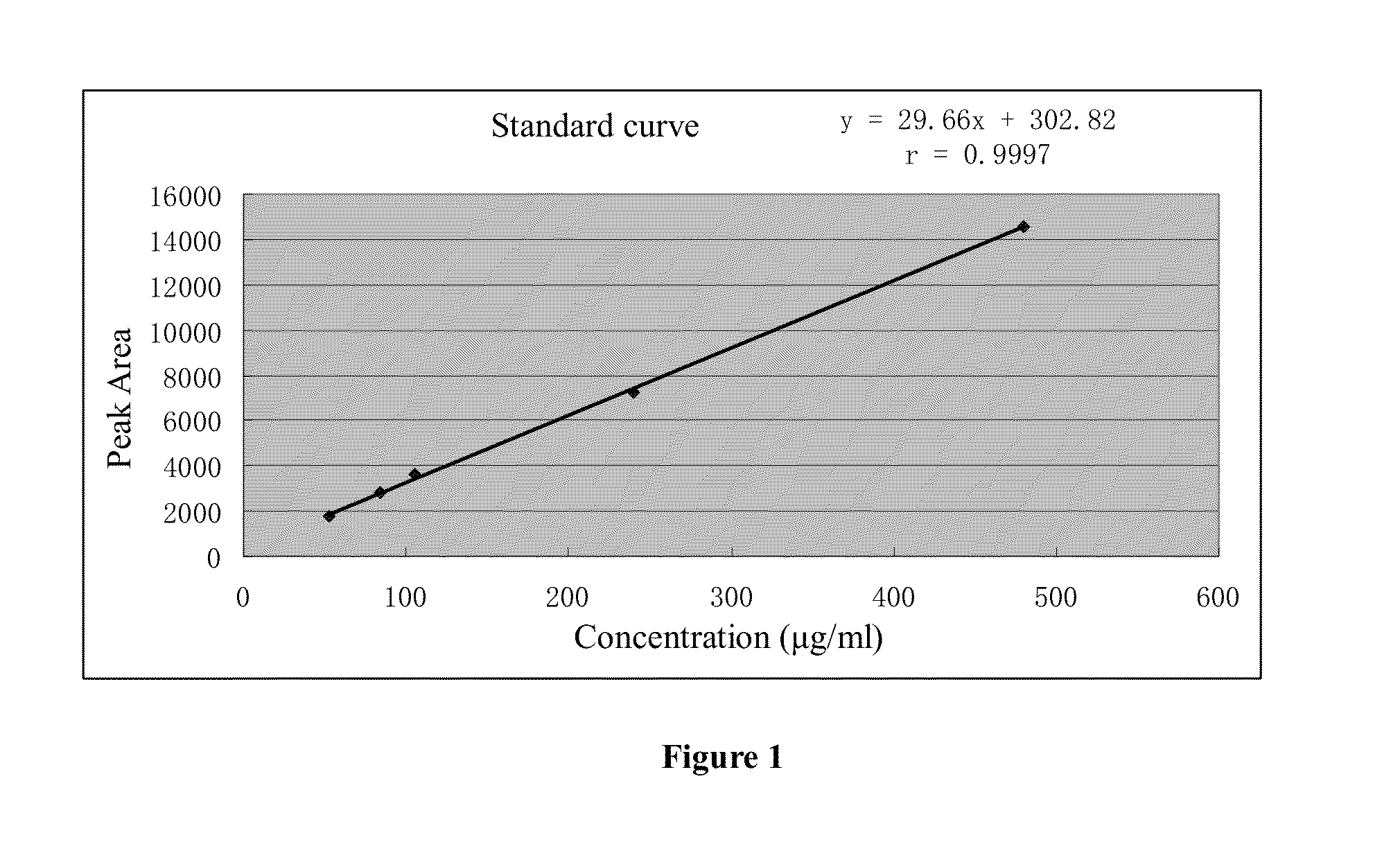
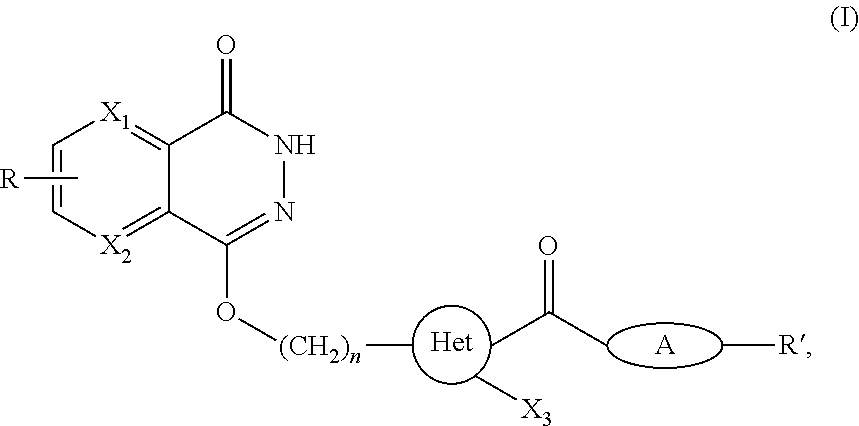
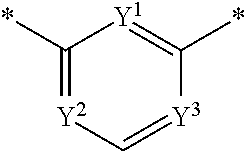
Poly (adp-ribose) polymerase inhibitor
a polymerase inhibitor and polymerase technology, applied in the field of poly (adpribose) polymerase inhibitors, can solve the problems of cell necrosis, cell death, and depletion of nadsup>, and achieve the effect of reducing the number of polymerase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0075]The followings further explain the general methods of the present invention. The compounds of the present invention may be prepared by the methods known in the art. The following illustrate the detailed preparation methods of the preferred compounds of the present invention. However, they are by no means limiting the preparation methods of the compounds of the present application.
[0076]Phthalic anhydride (1a) was reacted with hydrazine hydrate in the presence of glacial acetic acid to give phthalhydrazide (1b), which was then reacted with ethyl 3-chloromethylbenzoate (1c) in the presence of sodium hydride to give ethyl 3-((4-oxo-3-1,4-dihydro-phthalazin-1-yl-oxyl) methyl)benzoate (1d), after that, 1d was hydrolyzed into 3-((4-oxo-3,4-dihydrophthalazin-1-yl-oxy)methyl)benzoic acid (1e) under alkali condition, 1e finally condensated with R′ substituted nitrogen heterocycles, e.g. piperazine, piperidine, 4-hydroxypiperidine, tetrahydropyridine and 1,4-diazacycloheptane (homopiper...
preparation example 1
Intermediate Preparation Example 1
Synthesis of methyl 2-fluoro-5-hydroxybenzoate
[0091]
[0092]2-Fluoro-5-methoxybenzonitrile (3d, purchased from Alfa Aesar) (5 g, 33 mmol) was dissolved in hydrobromic acid (50 mL) and glacial acetic acid (30 mL), refluxed for 28 hours at 140° C., and then the reaction mixture was diluted with 100 mL ethyl acetate. The reaction mixture was then washed with 50 mL water and saturated sodium chloride solution successively for three times. The organic layers were combined and concentrated to give a crude product of 2-fluoro-5-hydroxybenzoic acid (3e), 2.88 g white solid (yield 56%). 1H NMR (600 MHz, DMSO-d6) δ 7.19-7.17 (m, 1H), 7.08-7.04 (m, 1H), 6.95-6.92 (m, 1H); ESI-MS m / z: calculated for 156.02. found 154.91 [M−H]+.
[0093]The obtained 2-fluoro-5-hydroxybenzoic acid (3e) (780 mg, 5 mmol) was dissolved in 100 mL methanol. Thionyl chloride (0.8 mL, 10 mmol) was added dropwise under the condition of ice bath (0° C.) and stirring, and then refluxed for two ...
preparation example 2
Intermediate Preparation Example 2
Preparation of 3-(((4-oxo-3,4-dihydrophthalazin-1-yl)oxy)methyl)benzoic acid (1e)
[0094]
[0095]Phthalic anhydride (1a, purchased from Shanghai Shaoyuan Co. Ltd.) (10 g, 67.6 mmol) was dissolved in glacial acetic acid (100 mL), hydrazine hydrate (20 mL) was added dropwise under the condition of ice bath (0° C.) and stirring, and then refluxed for two hours at 125° C. The reaction was monitored by TLC. The solution was cooled to room temperature, filtrated and washed with water until the pH of the filtrate was 6.0, and then dried to give phthalhydrazide (1b), 10.5 g white solid (yield 96%). ESI-MS m / z calculated for: 162.04. found: 173.15 [M+H]+.
[0096]Phthalhydrazide (1b) (10 g, 61.7 mmol) was dissolved in dried N,N-dimethylformamide (100 mL) in a round-bottom flask. Sodium hydride (1.63 g, 67.9 mmol) was added at 0° C., and then stirred for half an hour. Ethyl 3-chloromethylbenzoate (1c, purchased from Shanghai Shaoyuan Co. Ltd.) (13.49 g, 67.9 mmol) d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com