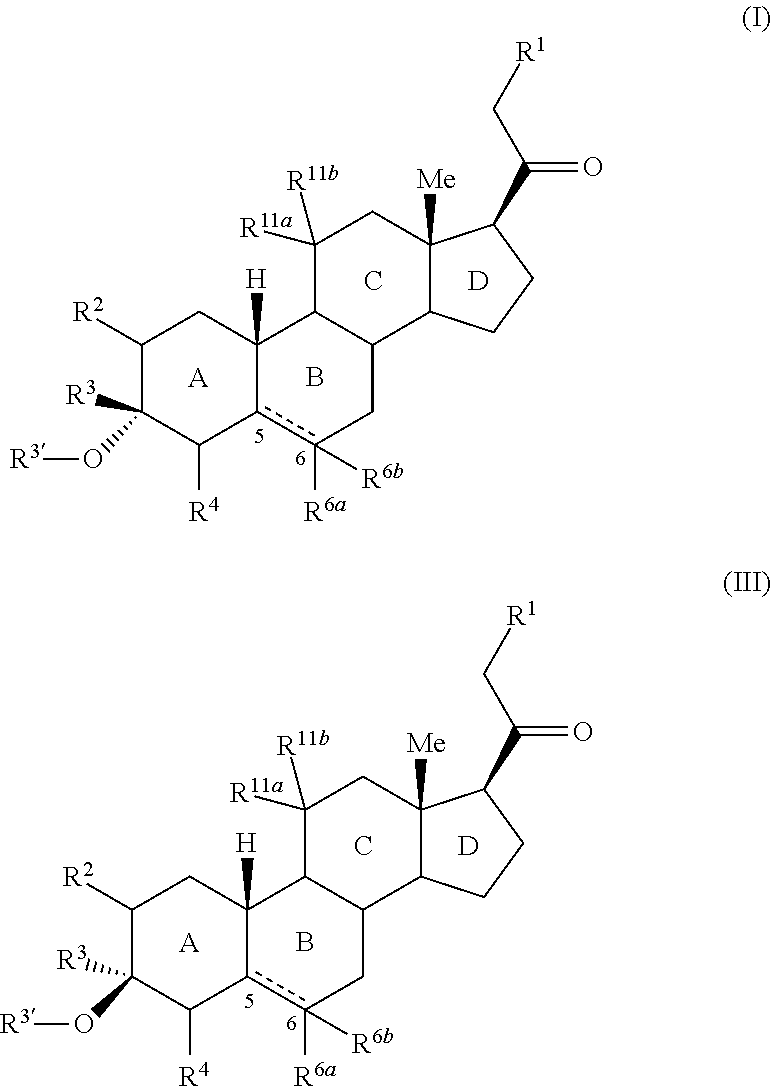
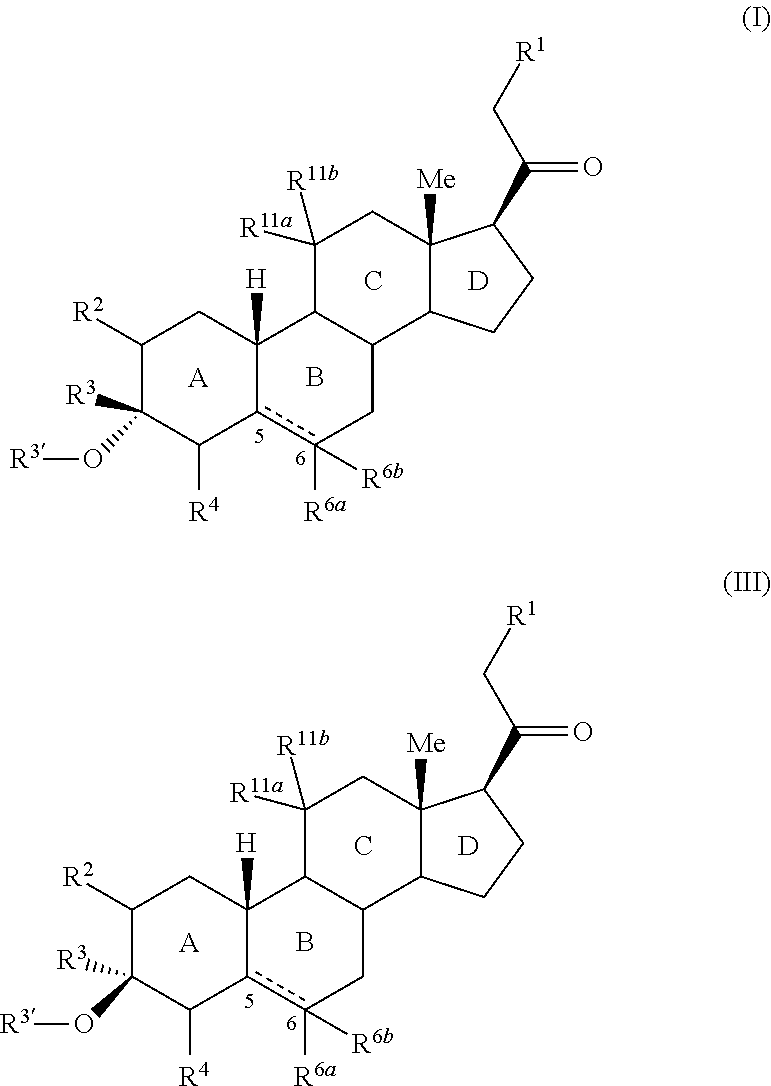
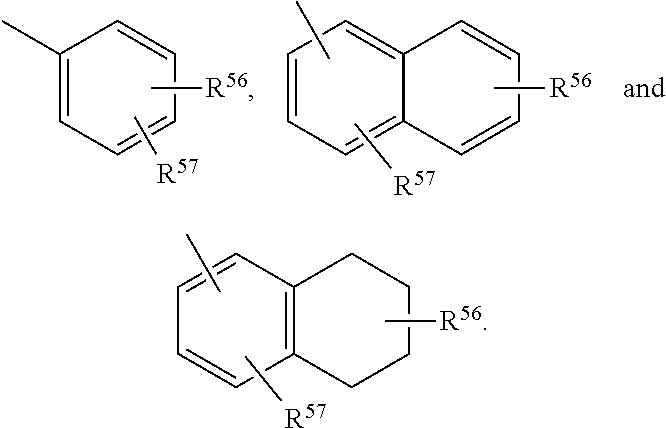
3,3 disubstituted 19-nor pregnane compounds, compositions, and uses thereof
a technology compound, which is applied in the field of disubstituted 19-nor pregnane compounds, compositions, and compositions, can solve the problems that progesterone is not consistently effective in the treatment of the aforementioned syndrome, and achieve the effects of preventing further metabolism, reducing the potential for oxidation to ketone, and reducing the risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compounds 8a / b, 9a / b, and 10a / b
[0358]
[0359]Synthesis of Compound 2.
[0360]Compound 1 (500 mg, 1.84 mmol) and 10% Pd / C (20 mg) in tetrahydrofuran (5 mL) and concentrated hydrobromic acid (0.02 mL) was hydrogenated with a hydrogen balloon. After stirring at room temperature for 24 h, the mixture was filtered through a pad of celite and the filtrate was concentrated in vacuo. Recrystallization from acetone to give compound 2 (367 mg, 1.34 mmol, 73%). 1H NMR (400 MHz, CDCl3), δ (ppm), 2.61-2.54 (m, 1H), 0.98 (S, 3H).
[0361]Synthesis of Compound 3.
[0362]To a solution of compound 2 (274 mg, 1 mmol) in methanol (4 mL) was added iodine (0.1 mmol). After stirring at 60 OC for 12 h, TLC showed no SM and the solvent was removed in vacuo. The crude product was dissolved in dichloromethane (20 mL) and washed with saturated NaHCO3 (15 mL), brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by chromatography on basic alumina (petroleum ether / ethyl acetate=9:1...
example 2
Synthesis of compounds 14a / b, 15a / b, and 16a / b
[0385]
[0386]Synthesis of Compound 11a and 11b.
[0387]To a solution of compound 5 (800 mg, 2.79 mmol) and PhSO2CF2H (540 mg, 2.79 mmol) in THF (25 mL) and HMPA (0.5 mL) at −78° C. under N2 was added LHMDS (4 mL, 1M in THF) dropwise. After stirring at −78° C. for 2 h, the reaction mixture was quenched with saturated aqueous NH4Cl solution (10 mL) and allowed to warm to room temperature then extracted with Et2O (20 mL×3). The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrate. The residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1) to give the mixture of compound 11a and 11b (700 mg). The mixture was further purified by chiral-HPLC to afford compound 11a (200 mg, t=4.31 min).
[0388]1H NMR (400 MHz, CDCl3), δ (ppm), 7.99-7.97 (d, 2H, J=7.6 Hz), 7.77-7.75 (m, 1H), 7.64-7.60 (m, 2H), 5.14-5.08 (m, 1H), 0.88 (s, 3H); compound 11b (260 mg, t=5.66 min). 1H N...
example 3
Synthesis of 6-difluoro Analogs
[0429]
PUM
| Property | Measurement | Unit |
|---|---|---|
| membrane voltage | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com