Pharmaceutical compositions of goserelin sustained release microspheres
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
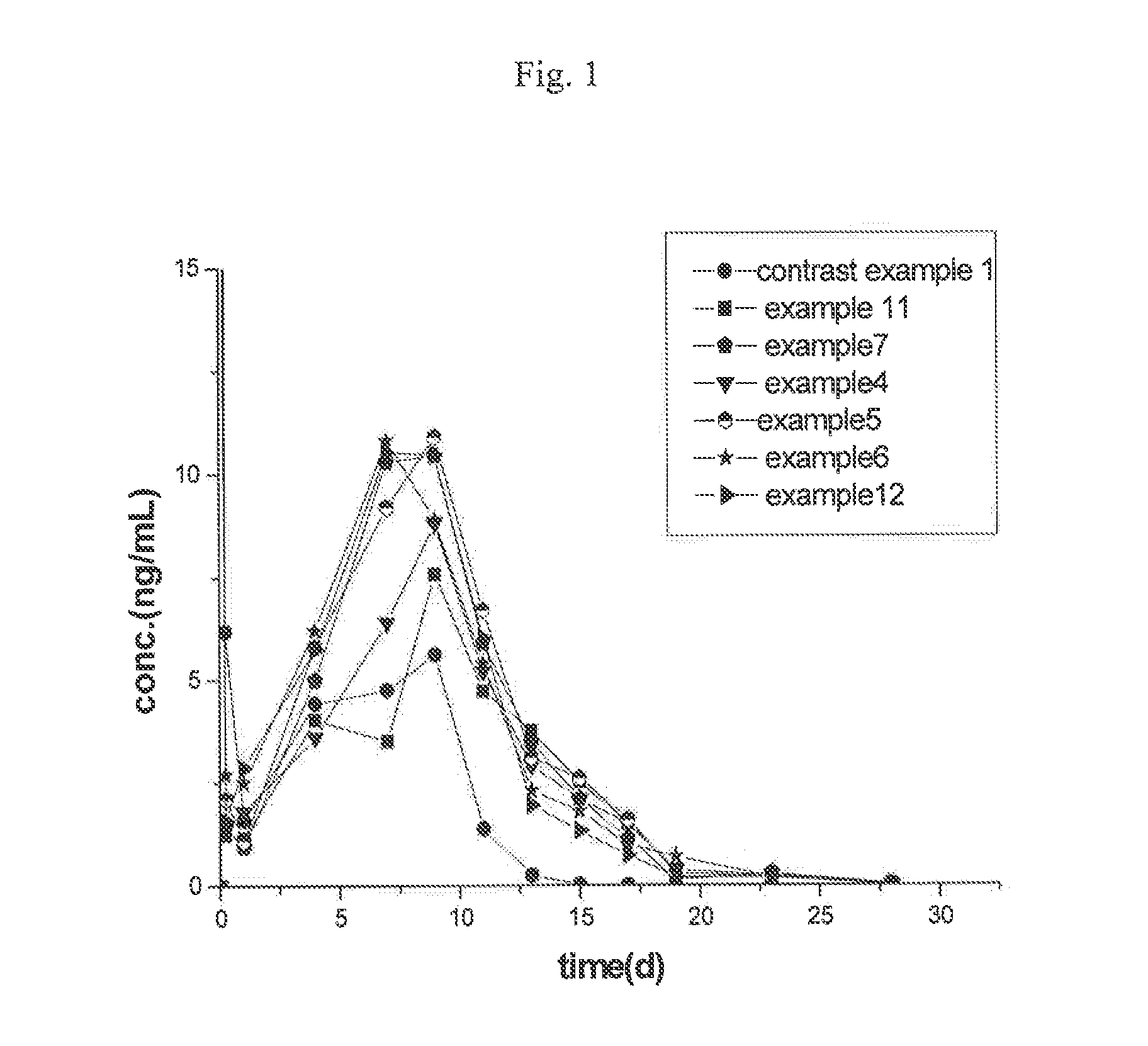
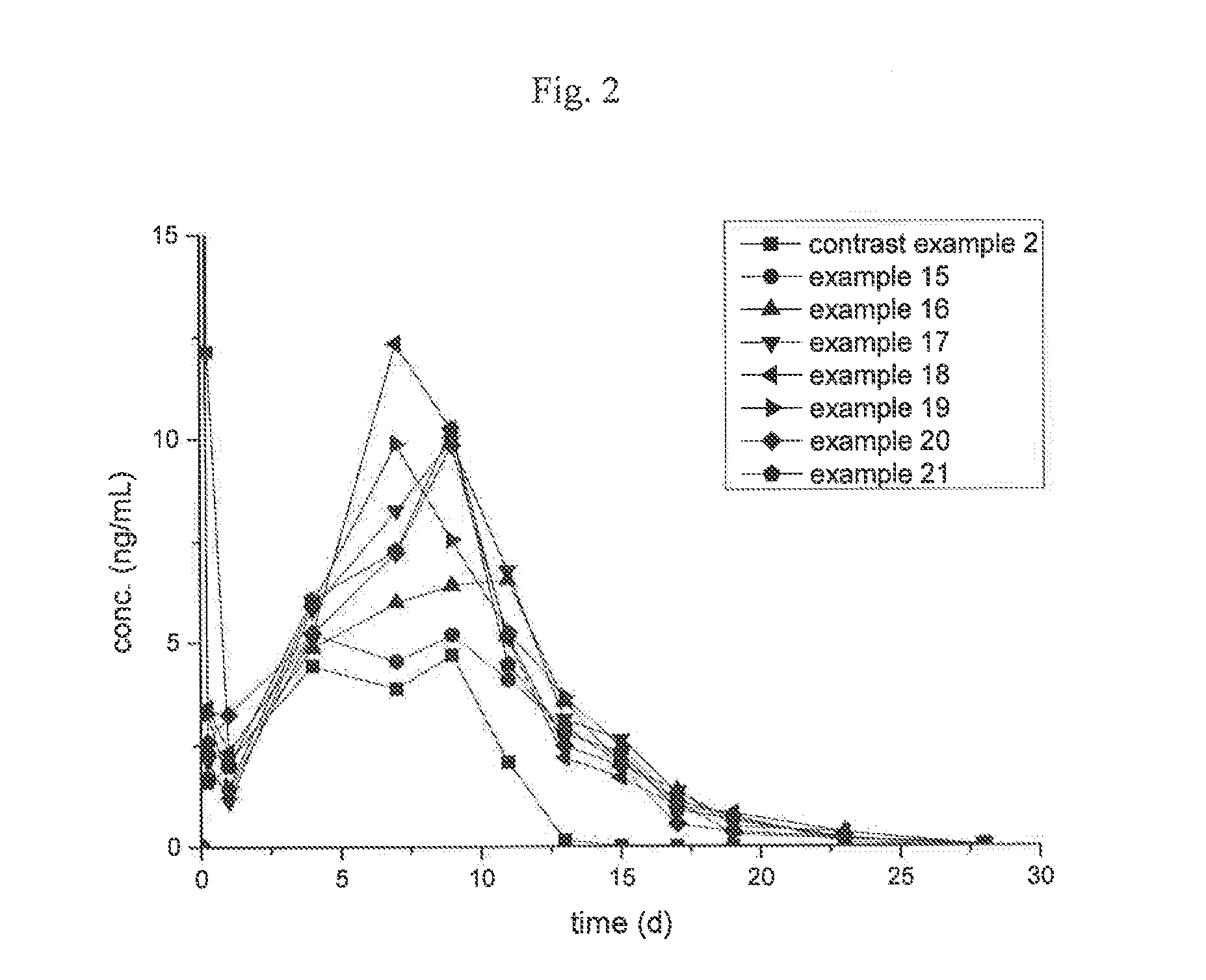
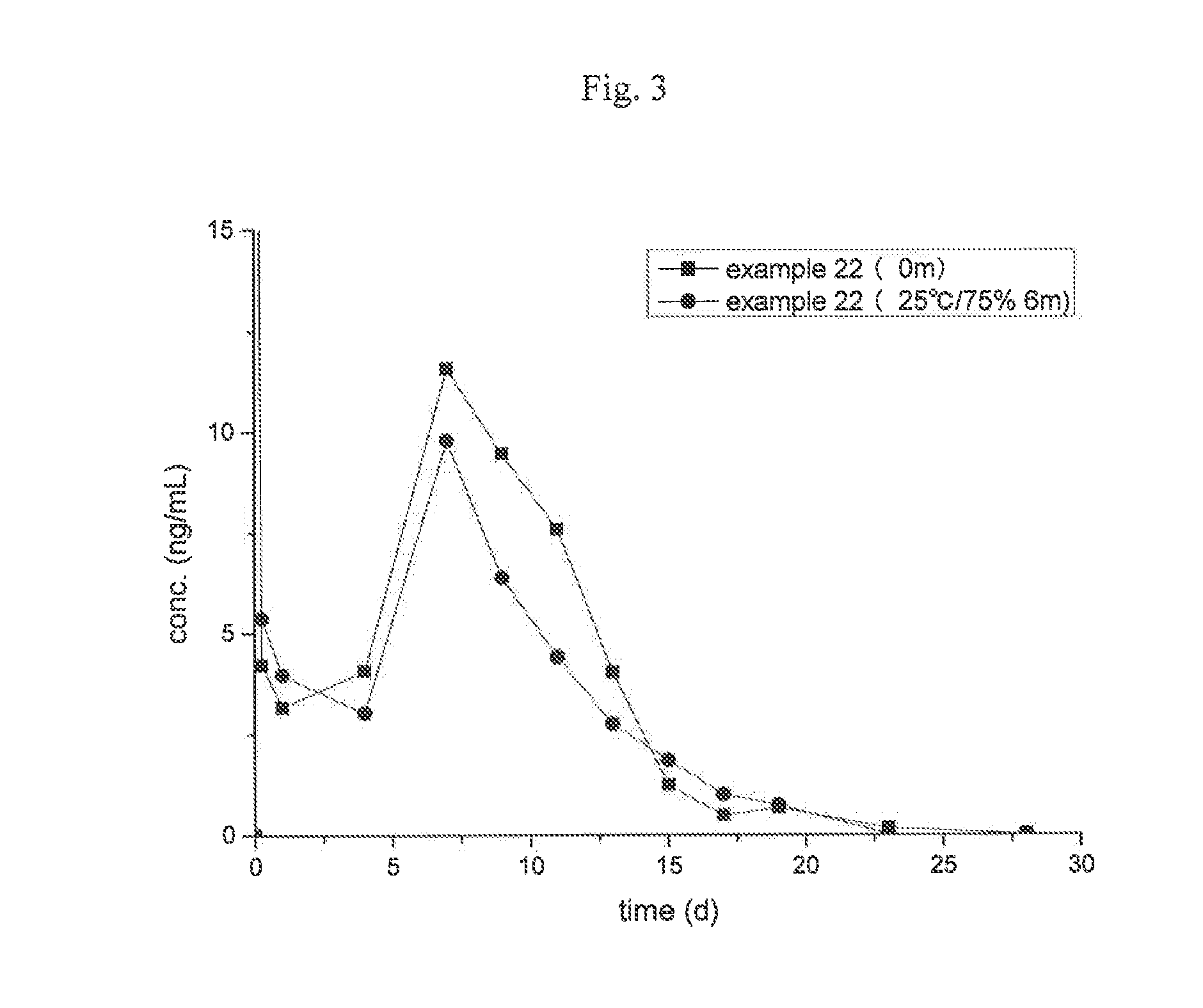
Examples
example 1
[0040]Appropriate amount of goserelin acetate and Poloxamer 188 were weighed and ball-mill mixed at frequency of 15 Hz for 5 min so as to obtain a mixture of solid powder. 430 mg mixture of goserelin and Poloxamer 188 (the measured amount of goserelin was 215 mg) was accurately weighed for later use. 1.721 g of PLGA (75 / 25, 0.35, 42,000) was weighed and dissolved in 10 ml of dichloromethane to form an oil phase; and then the pretreated drug mixture of solid powder was added into the oil phase, and subjected to emulsification in a high shear emulsifier (6,500 rpm, 3 min) so as to obtain a s / o primary emulsion. The primary emulsion was added into 1,000 ml of a 0.5% PVA solution at 6° C. under homogenization at 1,800 rpm, and then it was homogeneously emulsified for 2 min to obtain an S / O / W double emulsion. The double emulsion was stirred to volatilize and remove the organic solvent; the residue was washed and freeze-dried to obtain powdery microspheres. The microspheres had a drug loa...
example 2
[0041]The melting temperature of a melt extruder was set at 80° C. Appropriate amount of goserelin acetate and Poloxamer 407 were sifted and mixed. The mixture was fed into the cavity of the extruder. The stirring speed was set to n=60 and the stirring mixing time to 3 min. Then the valve handle was released to extrude the melted material, which was then allowed to become cool naturally. The material was ball-mill smashed for 2 min. 316 mg of the mixture of goserelin and Poloxamer 188 (the measured amount of goserelin was 158 mg) was accurately weighed for later use, 1.672 g of PLGA (25 / 75, 0.24, 25,000) was weighed and dissolved in 10 ml of dichloromethane to form an oil phase; and then the pretreated drug mixture of solid powder was added into the oil phase, and subjected to emulsification in a high shear emulsifier (6,500 rpm, 3 min) so as to obtain a s / o primary emulsion. The primary emulsion was added into 1,000 ml of a 0.5% PVA solution at 6° C. under homogenization at 1,800 r...
example 3
[0042]Appropriate amount of goserelin acetate and Poloxamer 188 were weighed and dissolved in water to form a clear solution, and then the solution was sprayed dried so as to obtain a mixture of solid powder. 47 mg spray-dried mixture of goserelin and Poloxamer 188 (the measured amount of goscrelin was 23 mg) was accurately weighed and put into a vial. 1.951 g of PLGA (65 / 35, 0.29, 32,000) was weighed and dissolved in 10 ml of dichloromethane to form an oil phase; and then the pretreated drug mixture of solid powder was added into the oil phase, and subjected to emulsification in a high shear emulsifier (6,500 rpm, 3 min) so as to obtain a s / o primary emulsion. The primary emulsion was added into 1,000 ml of a 0.5% PVA solution at 6° C. under homogenization at 1,800 rpm, and then it was homogeneously emulsified for 2 min to obtain an S / O / W double emulsion. The double emulsion was stirred to volatilize and remove the organic solvent; the residue was washed and freeze-dried to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com