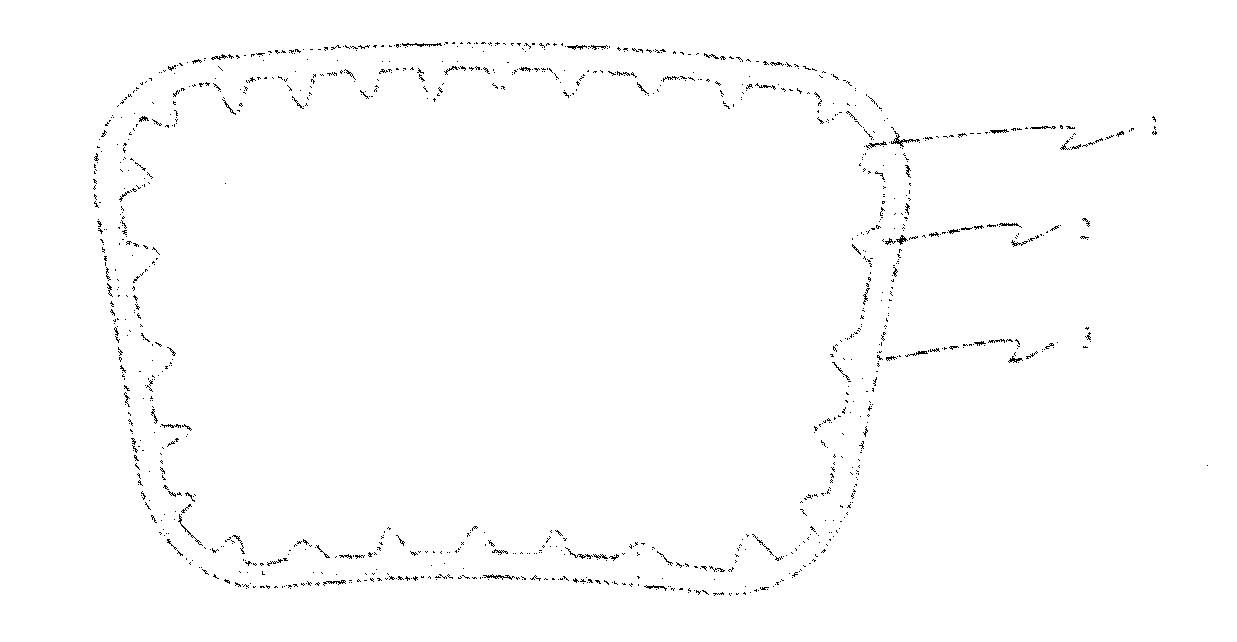
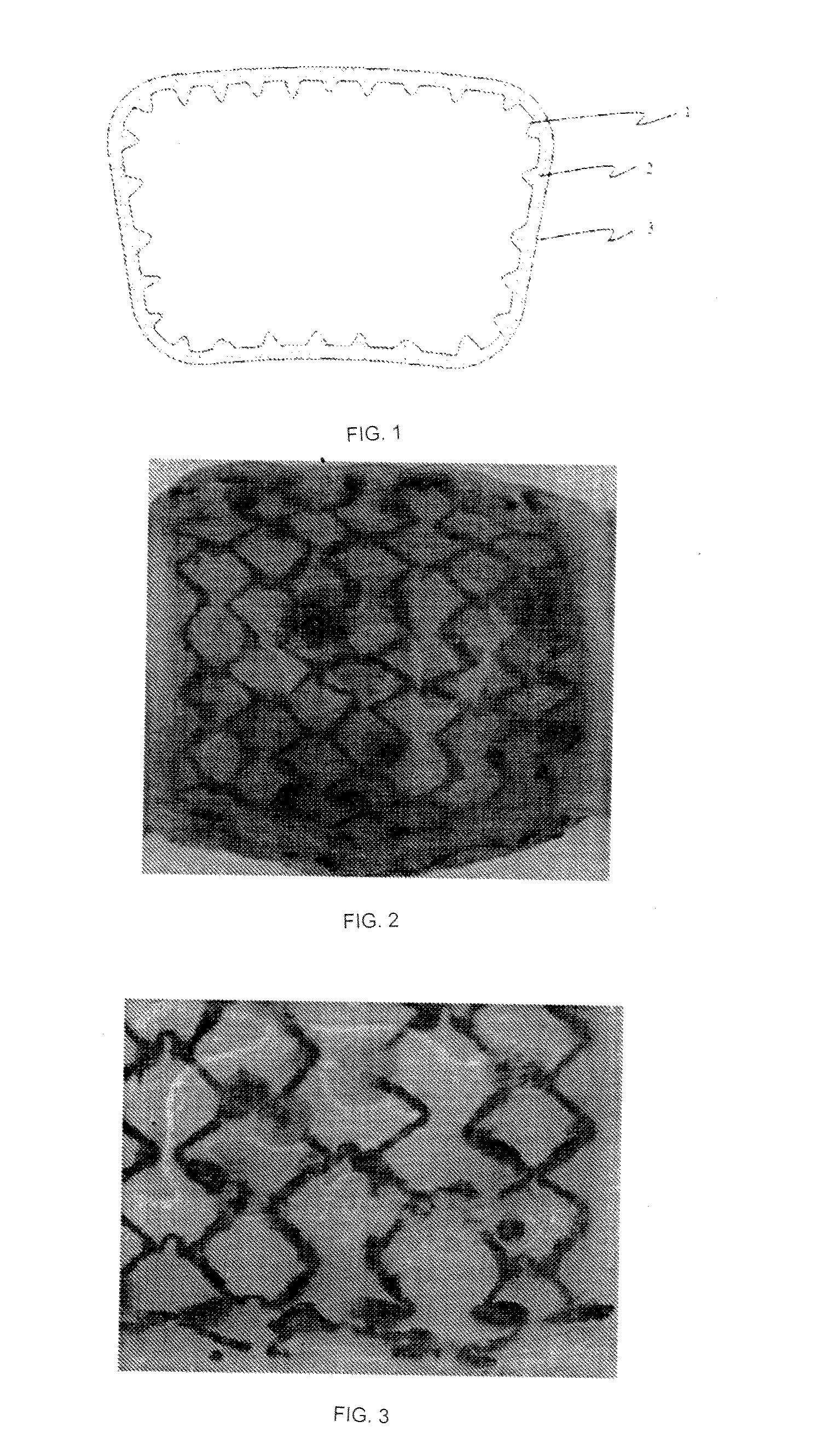
Absorbable Iron Alloy Stent
a technology of iron-based alloys and stents, which is applied in the direction of prosthesis, surgery, coatings, etc., can solve the problems of difficult to improve the corrosion rate of iron-based alloys, the need to accelerate the corrosion rate of iron, and the relative slow corrosion rate of pure iron, so as to avoid the risk of clinical air embolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0061]A pure iron stent comprises a pure iron substrate and a degradable polymer coating with which the surface of the pure iron substrate is coated, wherein the mass ratio of the pure iron substrate to the degradable polymer is 5:1. The degradable polymer is polyglycolic acid (PLA) with a weight average molecular weight of 200,000 and a polydispersity index of 1.8, and the wall thickness of the iron substrate is between 80 μm and 90 μm, and the thickness of the degradable polymer coating is between 15 μm and 20 μm. The stent was implanted into the abdominal aorta of a rabbit. The stent and the tissue in which the stent was placed were taken out at 3 months after the date of implantation, a radial support force test was carried out, and the test result that the radial support force was 70 kPa was obtained, indicating that the degradable polymer was well matched with the iron-based alloy substrate, and the early mechanical properties of the stent could be ensured; the periphery of th...
example 2
[0062]The surface of a bare nitrided pure iron stent (i.e., a nitrided pure iron substrate) of which the wall thickness is between 65 μm and 75 μm was uniformly coated with a 10 to 12 μm thick degradable polymer coating, wherein the mass ratio of the nitrided pure iron substrate to the degradable polymer is 25, and the degradable polymer coating is a poly(DL-lactic acid) coating with a weight average molecular weight of 100,000 and a polydispersity index of 3. The absorbable iron-based alloy stent was obtained after drying. The iron-based alloy stent was implanted into the coronary artery of a pig. At 3 months from the date of implantation, it was found that there was no difference between the surrounding area of the stent strut and the surrounding area of the stent strut at the beginning of implanting by OCT follow-up. The stent was taken out at 1 year after the implanting, the mass loss rate of the stent was 92 percent by a mass loss test, indicating that the stent completely corr...
example 3
[0063]The surface of a bare electrodeposited pure iron (550° C. annealing) stent (i.e., an electrodeposited pure iron substrate) of which the wall thickness is between 40 μm and 50 μm was uniformly coated with a 3 to 5 μm thick mixture coating of polycaprolactone (PCL) and paclitaxel, wherein the mass ratio of the electrodeposited pure iron substrate to the degradable polymer was 35:1, the polycaprolactone (PCL) was formed by mixing two kinds of polycaprolactones (PCL) with weight average molecular weights of 30,000 and 80,000 according to a ratio of 1 to 2, the polydispersity index of the mixed polycaprolactones (PCL) was 25, and the mass ratio of polycaprolactones (PCL) to paclitaxel was 2 to 1. An absorbable iron-based alloy stent was obtained after drying. The iron-based alloy stent was implanted into the abdominal aorta of a rabbit. The stent was taken out at a corresponding observation point in time, the surface of the stent was observed with a microscope, and the radial suppo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com