Microfluidic System and Method for Real-Time Measurement of Antibody-Antigen Binding and Analyte Detection
a microfluidic system and antibody technology, applied in fluorescence/phosphorescence, laboratory glassware, instruments, etc., can solve the problems of inability to monitor the continuous analyte immunoassay and pharmacokinetic characterization of biomolecules in real-time, laborious and time-intensive procedures, and methods that are impractical for real-time monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0138]Microfluidic Device Fabrication.
[0139]The polydimethylsiloxane (PDMS) microfluidic device was fabricated using well-established soft lithography method. Negative photo resist SU-8 2100 (MicroChem, Newton, Mass.) was spin-coated on Silicon wafers to a thickness of 150 μm, and patterned by exposure to UV light through a transparency photomask (CAD / Art Services, USA). PDMS (Sylgard 184, Dow Corning, MI) was mixed with the crosslinker (Sylgard 184 curing agent) in a ratio of 10:1, poured onto the photoresist patterns, degassed thoroughly and cured for 12 hours at 65° C. Next, the PDMS was peeled off the wafer and placed in oxygen-plasma chamber in order to bond with the glass slide. The device consisted of three inlets and two mixing channels. Tygon Micro Bore PVC Tubing 0.010″ ID, 0.030″ OD, 0.010″ Wall (Small Parts Inc., FL, USA) was connected to the channels and to 1 mL syringes. Syringe pumps (Harvard Apparatus, USA) were used to maintain a flow rate of 5 ...
example 2
Flow Dynamics in a Microfluidic Device
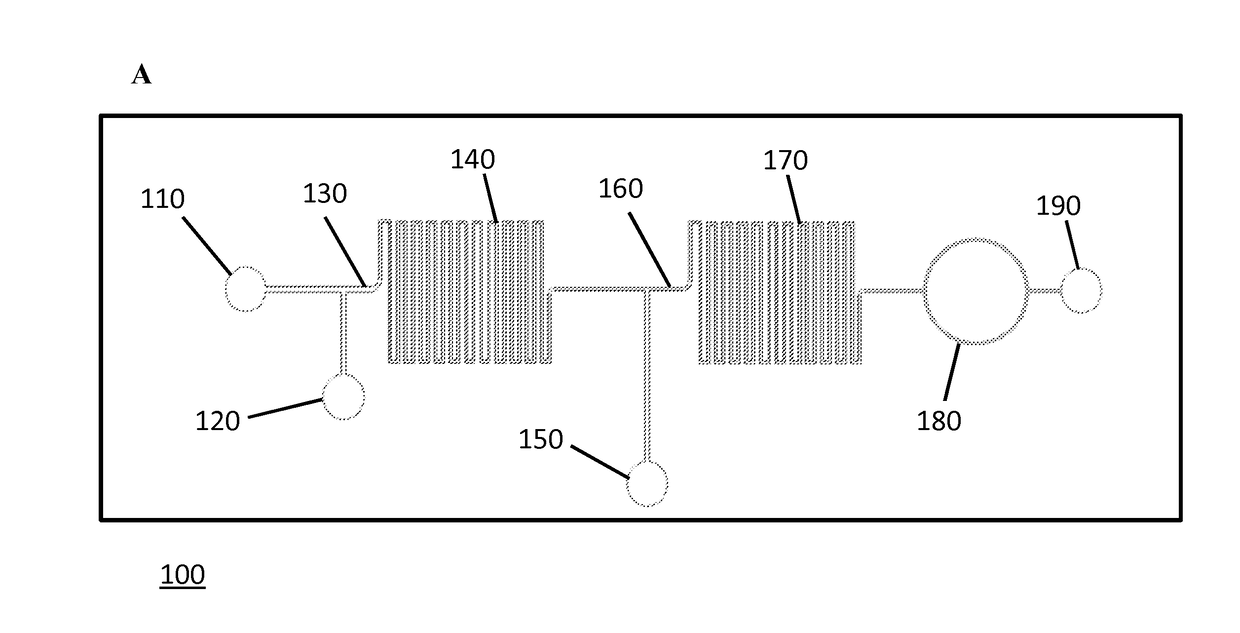
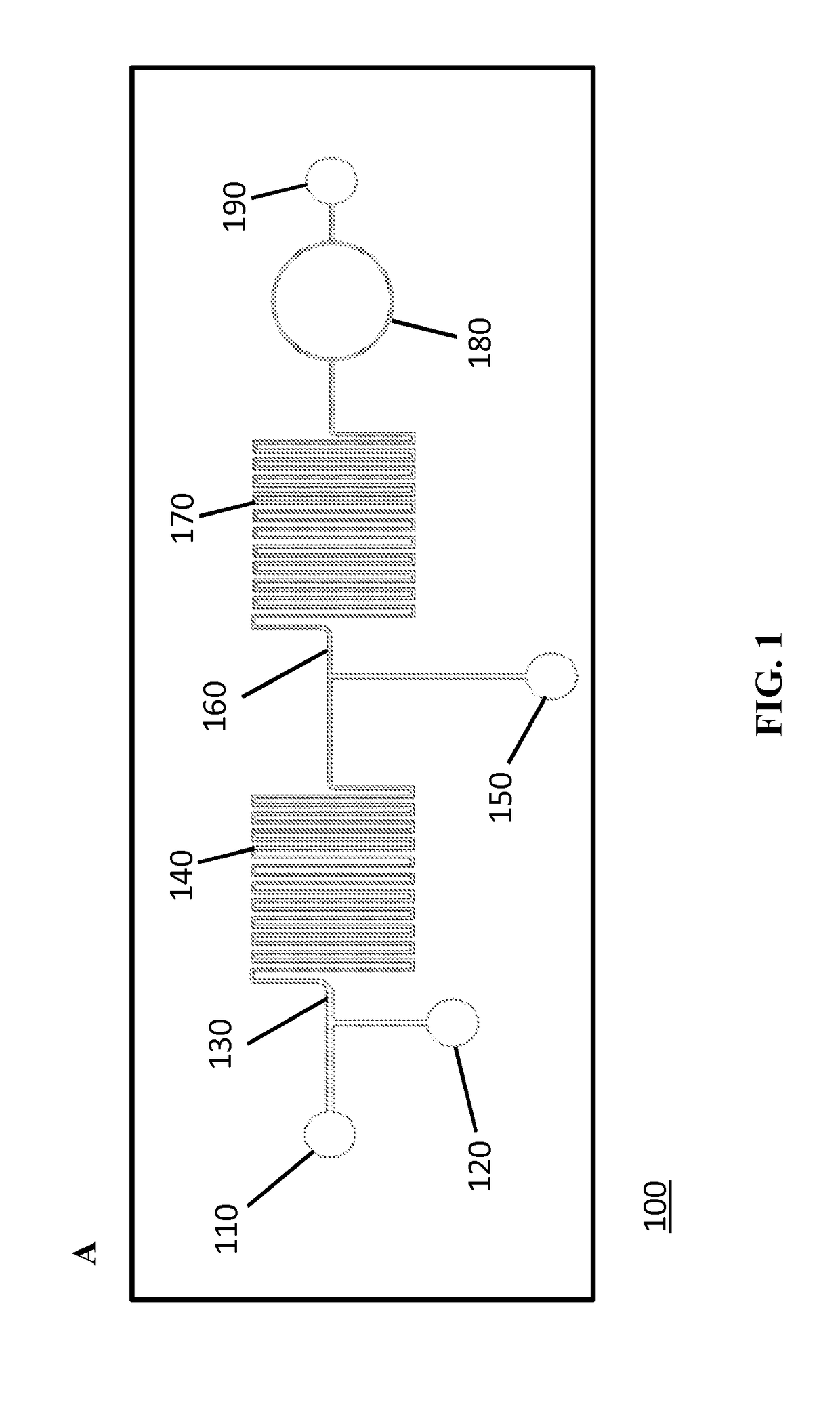
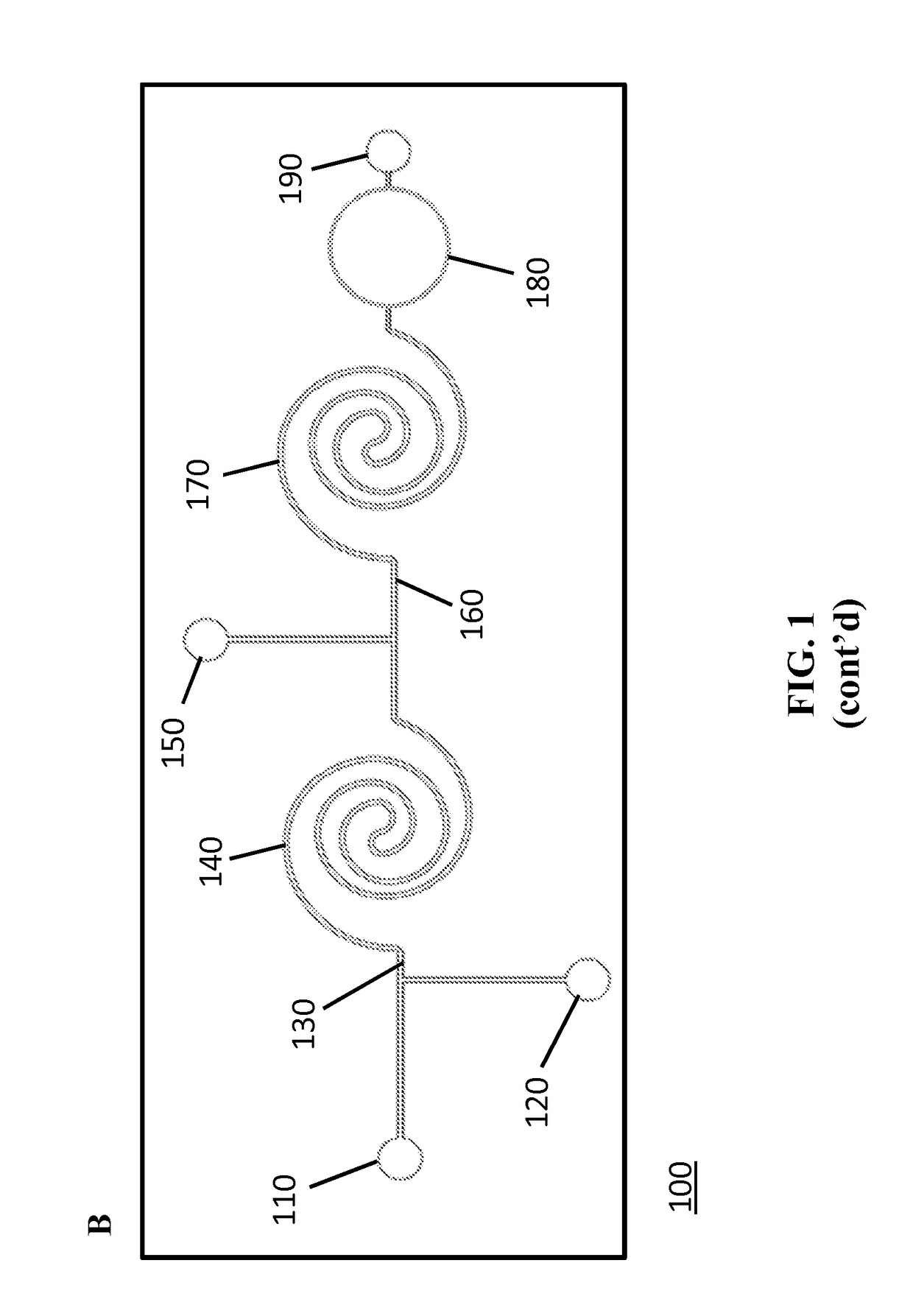
[0185]FIGS. 1A-1C schematically illustrates the developed flow-through LOC device. The device consisted of three inlets and two mixing regions. The inlets were connected with syringe pumps that were operated individually to obtain desired flow rates for the detection of microspheres downstream. First, a solution containing the analyte molecules was introduced into inlet 1 (110) and mixed with a suspension of functionalized microspheres that were introduced via inlet 2 (120) to capture the target analyte in the first mixing channel (140) (FIGS. 1A-1C). The specific interaction that occurs between the conjugated microspheres and the target analyte in the first mixing channel leads to the analyte recognition and capture. A detection (reporter) antibody against the analyte, conjugated with a specific fluorophore, was then introduced to the flow stream via inlet 3 (150) just before the second mixing channel (170). The sandwich complex formation, comp...
example 3
Anti-TNF-α Antibody Immunoassay
[0187]To demonstrate the real-time detection capabilities of the LOC device, efforts were focused on detecting anti-TNF-α antibody. FIG. 12A describes the microsphere-based assay that was introduced into microfluidic format for anti-TNF-α detection. Avidinilated microspheres were conjugated off-chip to biotinylated human TNF-α protein via avidin-biotin bridge (Konry et al., 2009; Diamdandis et al. 1991) as described in Example 1. Next, the generated anti-TNF-α microsphere-based sensors were introduced into the microfluidic device via inlet 2 while the analyte, mouse monoclonal anti-human TNF-α antibody, was introduced via inlet 1. The interaction of the two components resulted in the capture of anti-TNF-α antibodies by microsphere-based sensors in the first mixing channel of the device. Next, the detection antibody, FITC-labeled anti-mouse IgG, was introduced into inlet 3. The fluorescent signal on the microsphere sensor, generated by the conjugation o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| cross-sectional areas | aaaaa | aaaaa |
| fluidic path length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com