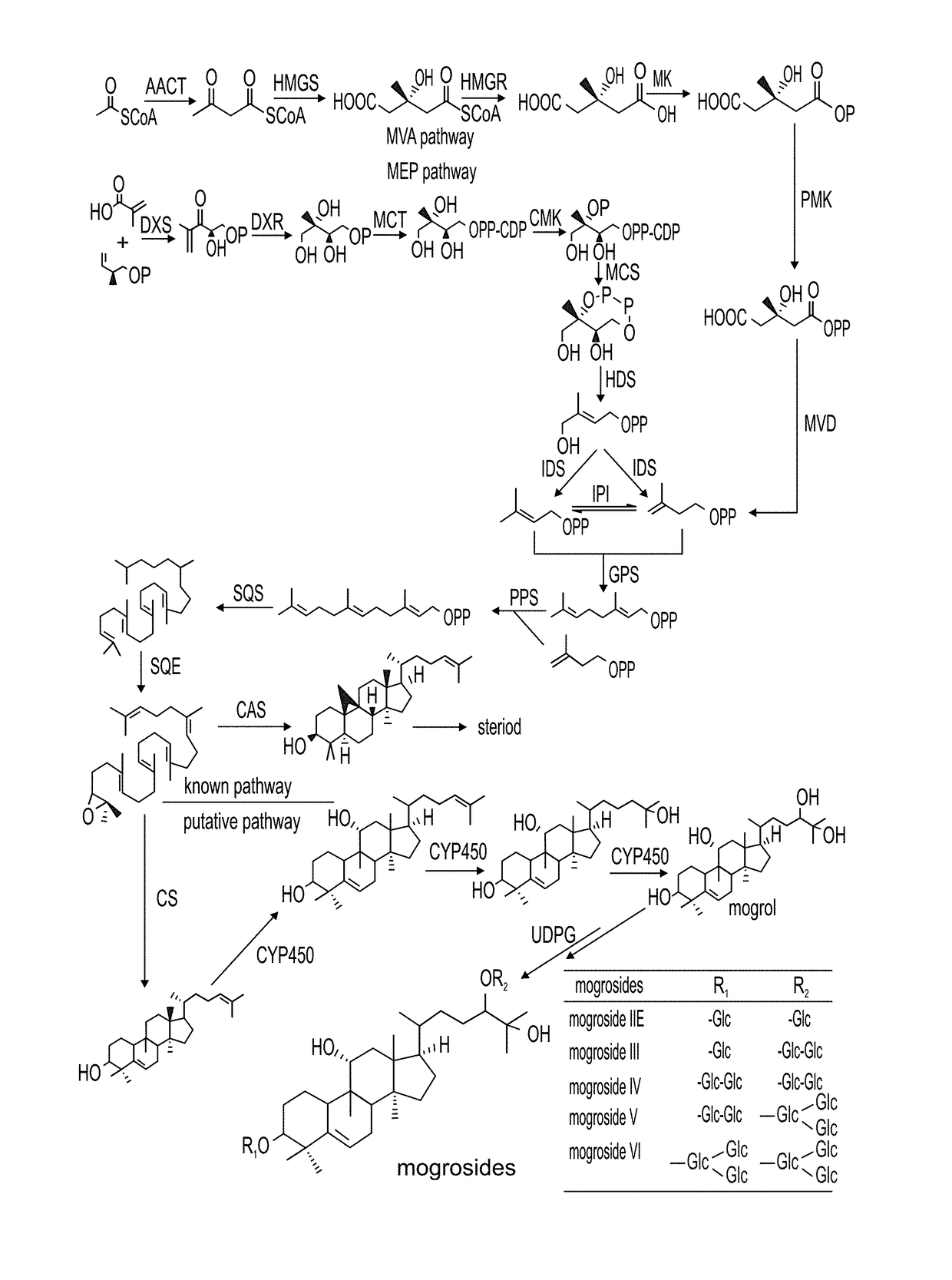
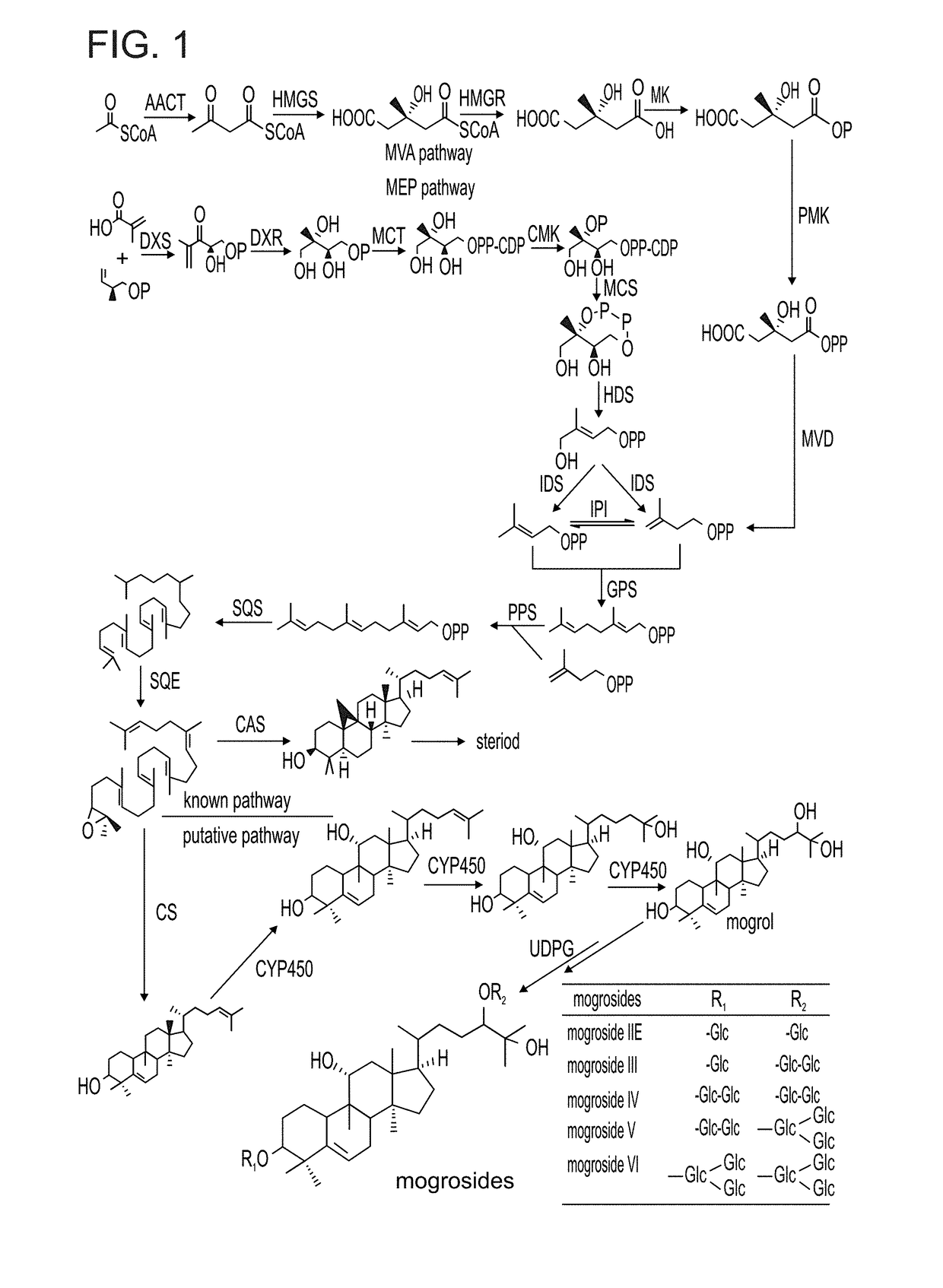
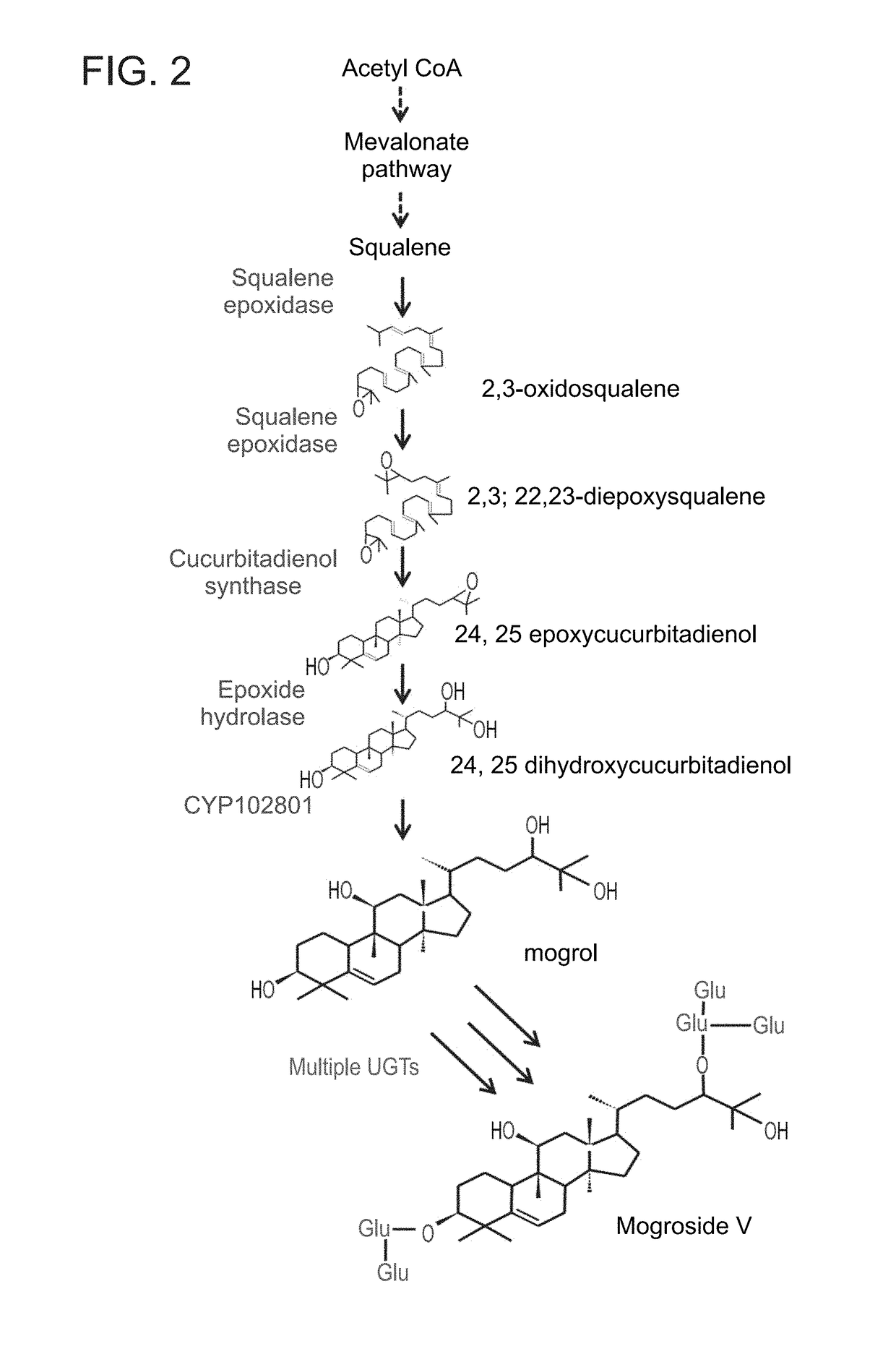
Methods of producing mogrosides and compositions comprising same and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Temporal Pattern of Mogroside Accumulation
[0336]Mogroside accumulation during development of the Siraitia fruit is shown in FIGS. 4A and 4B. Targeted metabolic profiling of Siraitia mogrosides during fruit ripening was carried out on methanolic extracts of the frozen powders and analyzed by HPLC with photodiode array and mass spec detection. Results reveal their unique temporal distribution. Mogrosides were limited to the developing fruit and were not observed in the root, stem or leaf tissue.
[0337]Already in the youngest stage of immature fruit analyzed, at 15 DAA (Days After Anthesis), the majority of the mogrols were present in the di-glucosylated form in which the C-3 and C-24 mogrol carbons are each mono-glucosylated. Non-glucosylated, mono-glucosylated or alternative M2 compounds, in which the second glucosyl moiety was present as a branched glucose on one of the primary glucose moieties, were not observed, indicating that the initial metabolic steps of mogroside glucosylation...
example 2
Identification of Siraitia Cucurbitadienol Synthase (SgCDS) as the Enzyme which Cyclicizes Both 2,3-Monoepoxysqualene and 2,3;22,23-Diepoxysqualene, Leading to, Respectively, Cucurbitadienol and 24,25-Epoxycucurbitadienol
[0343]The preferred substrate for the synthesis of the novel trans-C24,C25-dihydroxycucurbitadienol is 2,3;22,23-diepoxysqualene which is symmetrically epoxidated at both ends of the squalene molecule at the squalene numbered positions of C2,3 and C22,23 (FIG. 3). 2,3;22,23-diepoxysqualene is synthesized by the enzyme squalene epoxidase (SQE) which is ubiquitous in squalene metabolizing organisms, including the yeast strain GIL77. The yeast strain GIL77 is one of the strains in which the yeast gene erg7 encoding lanosterol synthase is mutated and non-functional, thereby making available the 2,3-epoxysqualene precursor to the cucrbitadienol synthase cyclization reaction and allowing for the synthesis of cucurbitadienol. This has previously been shown for the Cucurbit...
example 3
Identification of S. Grosvenorii Epoxy Hydratase Enzymes Catalyzing the Hydration of 24,25-Epoxycucurbitadienol in Mogrol Biosynthesis
[0347]In order to identify candidate Siraitia epoxy hydratase genes that may be involved in mogrol biosynthesis a detailed transcriptome analysis of 6 stages of developing Siraitia fruit was performed. The fruit stages were 15, 34, 55, 77, 93 and 103 days after fruit set, and used for the productions of transcriptome and mogroside metabolome that are described above. Data mining of Siraitia transcriptome led to the identification and isolation of 4 candidate epoxy hydratase enzymes (contigs 73966, 86123, 102640 and 28382) with high levels of expression early in fruit development (FIGS. 7 and 21-24).
[0348]The epoxy hydratase genes were expressed in GIL77 yeast, and the products assayed for production of 24,25-dihydroxycucurbitadienol from 24,25-epoxycucurbitadienol, the product of the previously described SgCDS reaction. FIGS. 8A and 8B show the effect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com